Materials and Structures for Li-air Batteries
- Category: Energy
- Tags: carl thompson, kathleen fitzgerald
Lithium-air batteries hold promise for the next generation of electric vehicles and other applications. By reacting oxygen directly with lithium ions to form Li2O2 on discharge, these batteries can achieve energy densities 3-5 times higher than current lithium-ion batteries[1]. However, a number of challenges exist for implementation, including poor rate capability, poor cyclability, high overpotentials upon charging, and electrode and electrolyte instability[2]. We seek to address these issues by developing new electrode materials and architectures.
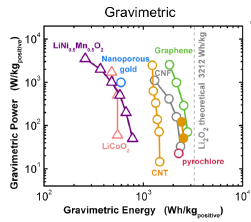
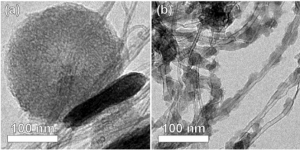
Aligned arrays of carbon nanotubes and nanofibers (CNT/CNFs) provide ideal conducting scaffolds for growth of Li2O2 particles, while the CNT/Fs themselves are low in mass and occupy a small volume fraction of the discharged electrode. We have grown aligned CNFs 5-10 nm in diameter on oxidized silicon substrates and de-laminated them in the form of cohesive carpets. These free-standing conducting carpets were then placed directly into lithium-air cells. We observed near-ideal gravimetric capacities (Figure 1) and high volumetric energy capacities. Control of particle morphology is critical for the high volumetric capacities needed for most applications. Two distinct types of particle morphologies were observed upon discharge, depending on the rate and depth of discharge[3]. At low charge rates or high depths of discharge, we observe the formation of large toroid particles. At high rates of discharge, we observe the formation of copious small particles (Figure 2). From x-ray diffraction and selected area electron diffraction, we determined that these are Li2O2 particles with predominantly (0001) surfaces[3].
Additionally, we have studied the stability of these carbon nanotube electrodes in a lithium-air cell[4]. We observed higher charging overpotentials as the cycle number increased. Using X-ray absorption near edge spectroscopy, we determined that Li2CO3 formed in increasing amounts upon cycling at the interface between lithium peroxide and the carbon nanotube [4]. We attribute poor cycling performance and higher charge overpotentials to this Li2CO3 formation, possibly due to reaction of Li2O2 with the CNTs. We are currently carrying out research to address this issue.
- Figure 1: A plot of the gravimetric power and energy of CNT and CNF batteries as compared to other lithium-air and lithium-ion cathodes and the theoretical limit of gravimetric energy[5].
- Figure 2: Lithium peroxide particles formed at (a) low charge rates or high depth of discharge, and (b) at high charge rates.
- R. R. Mitchell, B. M. Gallant, C. V. Thompson, and Y. Shao-Horn, “All-carbon-nanofiber electrodes for high-energy rechargeable Li–O2 batteries,” Energy & Environmental Science, vol. 4, no. 8, p. 2952, Aug. 2011. [↩]
- Y. C. Lu, B. M. Gallant, D. G. Kwabi, J. R. Harding, R. R. Mitchell, M. S. Whittingham, and Y. Shao-Horn, “Lithium-oxygen batteries: bridging mechanistic understanding and battery performance,” Energy and Environmental Science, vol. 6., p. 750-768, Jan. 2013. [↩]
- R. R. Mitchell, B. M. Gallant, Y. Shao-Horn, and C. V. Thompson, “Mechanisms of Morphological Evolution of Li2O2 Particles during Electrochemical Growth,” Journal of Physical Chemistry Letters, vol. 4., no. 7, p. 1060, Mar. 2013. [↩] [↩]
- B. M. Gallant, R. R. Mitchell, D. G. Kwabi, J. Zhou, L. Zuin, C. V. Thompson, and Y. Shao-Horn, “Chemical and Morphological Changes of Li–O 2 Battery Electrodes upon Cycling,” The Journal of Physical Chemistry C, vol. 116, no. 39, pp. 20800–20805, Oct. 2012. [↩]
- Y. C. Lu, B. M. Gallant, D. G. Kwabi, J. R. Harding, R. R. Mitchell, M. S. Whittingham, and Y. Shao-Horn, “Lithium-oxygen batteries: bridging mechanistic understanding and battery performance,” Energy and Environmental Science, vol. 6., p. 750-768, Jan. 2013. [↩]