Understanding and Controlling the Substrate Effect on Graphene Electron-transfer Chemistry via Reactivity Imprint Lithography
- Category: Materials, Nanotechnology
- Tags: michael strano, qing hua wang
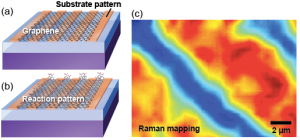
Figure 1: (a) Schematic of graphene supported on a chemically patterned substrate. (b) Schematic of reaction pattern in graphene induced by substrate. (c) Raman map of reaction intensity.
Graphene has exceptional electronic, optical, mechanical, and thermal properties, which provide it with great potential for use in electronic, optoelectronic and sensing applications[1]. The chemical functionalization of graphene has been investigated with a view to controlling its electronic properties and interactions with other materials[2]. Covalent modification of graphene by organic diazonium salts has been used to achieve these goals, but because graphene comprises only a single atomic layer, it is strongly influenced by the underlying substrate. In our work here[3], we show a stark difference in the rate of electron-transfer reactions with organic diazonium salts for monolayer graphene supported on a variety of substrates. Reactions proceed rapidly for graphene supported on SiO2 and Al2O3 (sapphire), but negligibly on alkyl-terminated and hexagonal boron nitride (hBN) surfaces, as shown by Raman spectroscopy. We also develop a model of reactivity based on substrate-induced electron–hole puddles in graphene and achieve spatial patterning of chemical reactions in graphene by patterning the substrate.
- A. K. Geim1 and K. S. Novoselov, “The rise of graphene,” Nature Materials, vol. 6, pp. 183-191, Mar. 2007. [↩]
- G. L. C. Paulus, Q. H. Wang, and M. S. Strano, “Covalent Electron Transfer Chemistry of Graphene with Diazonium Salts,” Accounts of Chemical Research, vol. 46, pp. 160-170, Sep. 2012. [↩]
- Q. H. Wang, Z. Jin, K. K. Kim, A. J. Hilmer, G. L. C. Paulus, C.-J. Shih, M.-H. Ham, J. D. Sanchez-Yamagishi, K. Watanabe, T. Taniguchi, J. Kong, P. Jarillo-Herrero, and M. S. Strano, “Understanding and controlling the substrate effect on graphene electron-transfer chemistry via reactivity imprint lithography,” Nature Chemistry, vol. 4, pp. 724-732, Aug. 2012. [↩]