Properties of Polymorphs of Belite
- Category: Energy, Materials, Nanotechnology
- Tags: can ataca, jeffert grossman
Portland cement clinker contains four major phases; alite (C3S) is the most dominant (50-70% by mass) and highly reactive with water while belite (C2S) constitutes 15-30% of normal cement clinkers and reacts slowly with water. The production temperature of C2S is lower than that of C3S, making the improvement of the reactivity of C2S phase to increase its use, which could result in lower energy requirements, a long-standing challenge. At ordinary pressures, thermal and X-ray measurements have reported five different polymorphs of belite, including Beta (β), Gamma (γ), and three Alpha (α) phases[1]. We applied first-principles fully quantum mechanical analytical techniques to model different polymorphs of belite. Combining these techniques with statistical analyses, we predict the energetically most favorable structures of β and γ polymorphs. We calculate the substitution and formation energies for most of the dominant impurities (Al, K, Fe, and Mg) and vacancy defects. We examine how each impurity defect affects the reactivity of the material. By calculating the surface energies for all adatoms (atoms adsorbed into the crystal structure) in all polymorphs, we aim to understand how adatoms and defects play a role in determining surface energies and crystal reactivity. The γ-C2S polymorph forms after cooling β-C2S below 500oC. The calculated cohesive energy of γ-C2S is the highest, establishing the stability of this polymorph. The charge density of γ-C2S in the lowest unoccupied molecular orbitals is lower than that of β-C2S, which signals lower reactivity. Our initial calculations demonstrate that adatoms influence the reactivity of γ-C2S more than that of β-C2S. Surface energies shown in Figure 1 for γ-C2S polymorphs are higher than those for β-C2S. However, after the addition of impurities in our simulations, the calculated surface energies of γ-C2S polymorphs decreased significantly. We concluded that alkali metal impurities cause the low reactivity.
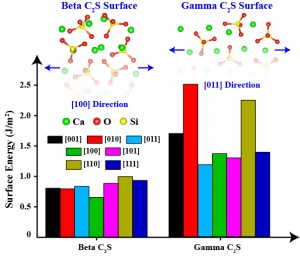
Figure 1: Energetics of Beta and Gamma C2S surfaces. The least energetic surface structures are indicated.
- H. F. W. Taylor, Cement Chemistry,London, Thomas Telford Publishing, 1997. [↩]