Non-Wetting Droplets on Superheated Textured Hydrophilic Surfaces
- Category: Nanotechnology
- Tags: evelyn wang, solomon adera
Engineered surfaces have received significant interest for a wide range of applications including microfluidics, drag reduction, self-cleaning, water harvesting, anti-corrosion, anti-icing, and thermal management. Significant efforts have focused on using chemical functionalization to change the wettability of such surfaces from superhydrophobic to superhydrophilic. In this work, we demonstrate that we can achieve non-wetting water droplets on superhydrophilic surfaces via evaporation. We demonstrate that when a wall superheat is below a critical superheat, the droplet spreads spontaneously following surface irregularities with near zero contact angle. However, when the superheat is above this critical superheat, the droplet rests on top of the structured surface without wetting with apparent contact angles as high as 160˚ due to evaporation. We studied this evaporation-induced non-wetting behavior of droplets by fabricating silicon micropillar arrays in a square pattern with pillar diameter (D), height (H), and pitch (L) ranging from 3-9 µm, 15-40 µm, and 10-30 µm, respectively. These samples were experimentally investigated at superheated conditions near the critical superheat to identify the effect of pillar array geometry (D, H, and L) and droplet size on the wetting behavior of droplets. A one-dimensional force balance model is developed that agrees reasonably well with experimental data. This phenomenon is distinct from the widely researched Leidenfrost and offers an expanded parametric space for fabricating surfaces with desired temperature-dependent wettability. Moreover, the framework can be extended to investigate the effect of pillar array geometry and material properties for the design of superhydrophobic surfaces at elevated temperatures.
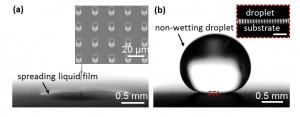
Figure 1: Effect of wall temperature on the wetting state of a water droplet. (a) Due to roughness-enhanced wettability, a water droplet deposited on a superhydrophilic surface at 120 °C spontaneously spreads into a thin film and wets the surface following surface irregularities with a near zero contact angle. The inset shows the scanning electron micrograph (SEM) image of the microstructured surface (D = 6.6 μm, H = 18.3 μm, and L = 20.0 μm) acquired at 10˚ inclination. (b) A similar size droplet at a higher wall temperature of 160 °C did not wet the same superhydrophilic surface; instead it rests on top of the structured surface forming a Cassie-like droplet. The inset shows a magnified view of the boxed section near the droplet base of a similar experiment indicating that the droplet remained in contact with the pillar tops. The scale bar in the inset represents 100 μm.