Microfluidic Electronic Detection of Protein Biomarkers
- Category: Electronic Devices, MEMS & BioMEMS
- Tags: dan wu, joel voldman
Immunoassays use antibodies to detect protein biomarkers, with a substantial global market and significant importance for clinical practice. However, traditional immunoassays are performed in centralized laboratories using optical detection methods, which means that results take days and cannot be highly multiplexed, in turn increasing patient visits, healthcare costs, and decreasing healthcare outcomes. In our project, we are developing an all-electronic immunoassay. All-electronic immunoassays have three major benefits: 1) we can achieve high-throughput immunoassays, allowing us to detect over 100 proteins in a single sample and potentially even to measure all approved protein biomarkers; 2) we can reduce cost by taking advantage of decreasing costs of silicon electronics; and 3) patients can get results before meeting with their physicians, since impedance detection is much faster than optical readout.
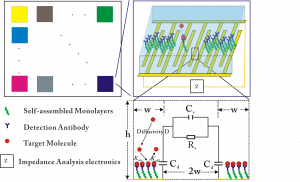
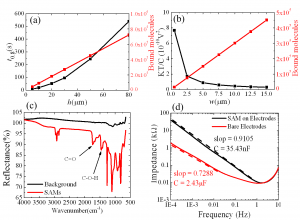
The basic idea of an all-electronic biosensor is that by looking into the impedance change caused by association of antigens to antibodies, we can determine if certain antigens are present in the blood. Our biosensor is illustrated in Figure 1. To facilitate the sensor design, we determined three parameters (detection sensitivity, response time and sensor dimension) to characterize the performance of the sensor. We built two models to gain insight into the relationship between the sensor design and performance. The transport model of molecular binding indicated that the response time is determined mainly by the height of the channel, and sensors with higher channels need more time to reach equilibrium but can have better signal-to-noise ratio (Figure 2a). The electric model of impedance change shows that the interfacial impedance determines the electric readout and that sensors with wide electrode can offer good signal-to-noise ratio but end up with large size (Figure 2b). Therefore, the three key performance parameters cannot be achieved simultaneously, and the sensor design needs to be optimized.
Another critical issue is chemical modification of the electrode’s surface; the goal is to eventually immobilize probe proteins on the electrodes. To this end, we built alkanethiol self-assembled monolayers (SAMs)as the links between gold electrodes and probe proteins. Since creating SAMs is the first step of surface modification, it is critically important to have successful SAMs. We used different surface characterization techniques, including Fourier transform infrared spectroscopy and impedance spectroscopy. Both methods showed that the self-assembled monolayers have been successfully built (Figures 2c and 2d).
- Figure 1: (a) The illustration of multiplexed all-electronic biosensors; (b) The illustration of one element of the sensor; and (c) The cross-section of one element.
- Figure 2: (a) The relationship between channel height and response time predicted by the transport model of molecule binding; (b) The relationship between electrode width and signal-to-noise ratio predicted by the electric model of impedance change; (c) Characterization of SAMs by FTIR. The two peaks corresponding to CO and COH bond are from the COOH group of SAMs; and (d) Characterization of SAMs by impedance spectroscopy. The increase in impedance is consistent with the fact that SAMs increase the thickness of interfacial capacitor.