Mechanical Pressure-induced Solid State Solvation in Organics Thin Films
- Category: Materials, Optics & Photonics
- Tags: vladimir bulovic, wendi chang
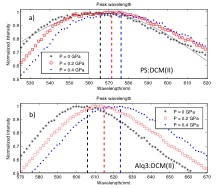
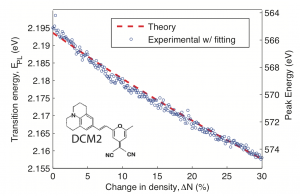
Significant technological progress of organic semiconducting structures has led to their commercialization in the form of organic LED displays, solid state lighting, and thin film photovoltaics. A guest:host doping system is often employed, and dicyanomethylene (DCM) class dyes are used extensively as the dopant molecules in both fundamental studies and applications[1],[2]. The solvation effect describes the physical reorientation of surrounding solvent molecules due to a change in solute dipole moment during transition, affecting the excitonic energy states of the emitting solute dye molecules. This local dielectric effect has been extensively studied in liquid state[3] and has been reported in molecular doping studies in solid state[4],[5]. In this work we demonstrate and numerically quantify a new method of probing dielectric-dependent excitonic-energy-shifts though application of pressure. Thin films of polystyrene (PS) and Alq3 doped with laser dye DCM(II) showed bathochromic shift in photoluminescence (PL) under increasing pressure, as shown in Figure 1. Films of 5% doped PS:DCM(II) showed peak energy shifts up to 40 meV under pressure change of 0.4 GPa, which when fitted to solid state solvation theory (Figure 2)[5],[6], suggests a PS elastic modulus of 1.2±0.6 GPa. Similarly, films of 1% doped Alq3:DCM(II) under pressure showed peak energy shifts up to 50meV, suggesting an Alq3 elastic modulus of 0.9±0.4 GPa. The results demonstrate the first numerically quantified solid-state solvation effect due to pressure. This ability to tune exciton energy using an external parameter creates a novel method to explore applications such as controlling exciton diffusion and optimizing optoelectronic device.
- Figure 1: Spectral shift in PL in response to pressure applied on a thin film with pressure, indicated in legend, of: (a) .5% doping of DCM(II) in PS and (b) 1% doping of DCM(II) in Alq3. The black line indicates a typical Gaussian fit of the peak PL of PS:DCM(II) film under no pressure. The maximum of the peak wavelength as computed from fitting is indicated for each PL as a corresponding dotted line.
- Figure 2: Fitting experimental results of PS;DCM(II) peak energy shift as function of changed molecular packing density or dielectric susceptibility. Red dashed line indicate the OLM theory of DCM(II) with initial dielectric constant of 2.44 (x0 = 1.44) in polystyrene, and blue points indicate the fitted peak wavelength from the pressure experiment using elastic modulus as a fitting parameter. The plot shows good agreement for a PS elastic modulus of 1.2 0.6GPa.
- M. Swoboda, Organic Microcavity Laser Dynamics: Fundamentals and Experiments on a DCM:Alq3 Guest: Host-composite System: VDM Verlag, 2008. [↩]
- Z. Guo, W. Zhu, and H. Tian, “Dicyanomethylene-4H-pyran chromophores for OLED emitters, logic gates and optical chemosensors,” Chemical communications (Cambridge, England), vol. 48, no. 49, pp. 6073–84, June 2012. [↩]
- C. Reichardt, Solvents and Solvation Effects in Organic Chemistry: VCH, 1990. [↩]
- V. Bulovic, R. Deshpande, M. E. Thompson, and S. R. Forrest, “Tuning the color emission of thin film molecular organic light emitting devices by the solid state solvation effect,” Chemical Physics Letters, no. 308, pp. 317–322, 1999. [↩]
- C. F. Madigan and V. Bulović, “Solid state solvation in amorphous organic thin films,” Physical Review Letters, vol. 91, no. 24, p. 247403, 2003. [↩] [↩]
- L. Onsager, “Electric Moments of Molecules in Liquids,” Journal of the American Chemical Society, vol. 58, pp. 1486–1493, 1936. [↩]