Improving Water Desalination Capabilities through the Use of Engineered Nanostructured Materials
- Category: Nanotechnology
- Tags: evelyn wang, tom humplik
Manipulation and control of transport at nanometer length scales offers new opportunities to enhance the performance of water desalination systems[1]. We report on two projects that utilize nanometer and sub-nanometer sized pores to better separate salt from water.
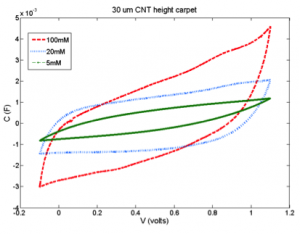
Capacitive deionization (CDI), a method that captures ions in the electric double layer (EDL), can be used to desalinate brackish waters[2]. Similar to supercapacitors, ideal CDI electrodes should have a large electrolyte-accessible specific surface area available for ion adsorption. To study the role of porous geometries in ion adsorption, we developed carbon nanotube (CNT) carpet electrodes, using an Au-Au self-diffusion bond to transfer vertically aligned CNT carpets onto an electrochemically-compatible substrate[3]. We are investigating the role of ion solution concentration on the capacity of the electrodes. Preliminary results show a three-fold increase of capacitance from 5mM solution (brackish water limit) to 100mM solution (Figure 1). At present, we are investigating the effect of pore lengths on capacitance and ion adsorption.
Additionally, we are fabricating size-exclusion based reverse osmosis (RO) membranes, which allow for water permeation while perfectly rejecting solvated salt ions. To achieve this, we are utilizing the ~5.5 Å pore size of MFI-type zeolites, which falls between the size of a water molecule (~3 Å) and a solvate salt ion (~7-8 Å)[4]. We investigated the permeability of water across the pores of these zeolites using water adsorption analysis. By varying the compositional silicon to aluminum (Si/Al) ratio of the zeolite, we can control the affinity of water to the zeolite. Decreasing the Si/Al ratio increases the amount of water within the zeolite pores at reverse osmosis operating pressures. However, decreasing this ratio has a significant detrimental effect on the permeability. Figure 2, which shows the water permeability as a function of the inverse Si/Al ratio, reveals that the higher Si/Al ratio zeolites exhibit enhanced water permeability, which is within an order of magnitude of current state-of-the-art RO membranes. We are in the process of fabricating defect-free zeolite-based membranes to further investigate the water and salt permeability. The authors would like to thank the King Fahd University of Petroleum and Minerals in Dhahran, Saudi Arabia, for funding the research reported in this paper through the Center for Clean Water and Clean Energy at MIT and KFUPM.
- Figure 1: Cyclic voltammetry scan at 500mV/s of CNT electrode in NaCl solution. Increasing NaCl concentration, increases electrical capacitance, and decreases ion adsorption and removal from water
- Figure 2: Permeability of water within zeolites as a function of the inverse Si/Al ratio. Increasing the Si/Al ratio increases the permeability of water through the zeolite pores. The polymeric RO permeability values can be found in[5].
- T. Humplik, J. Lee, S. C. O’Hern, B. A. Fellman, M. A. Baig, S. F. Hassan, M. A. Atieh, F. Rahman, T. Laoui, R. Karnik, and E. N. Wang, “Nanostructured materials for water desalination,” Nanotechnology, vol. 22, no. 29, p. 292001, Jun. 2011. [↩]
- Y. Oren, “Capacitive deionization (CDI) for desalination and water treatment – past, present and future (a review),” Desalination, vol. 228, no. 1, pp. 10–29, 2008. [↩]
- R. Enright, R. Mitchell, H. Mutha, C. Lv, M. Christiansen, C. V. Thompson and E. N. Wang, “Diffusion-bonded CNT carpets for fundamental CDI studies,” MRS Symposium Proceedings, vol. 1407, Boston, MA, Dec. 2011. [↩]
- S. C. Maroo, T. Humplik, T. Laoui, and E. N. Wang, “Water Transport in Sub-nanometer MFI Zeolites for Efficient Water Desalination,” in Proc. ASME 2012 3rd Micro/Nanoscale Heat & Mass Transfer International Conference (2012). [↩]
- T. Humplik, J. Lee, S. C. O’Hern, B. A. Fellman, M. A. Baig, S. F. Hassan, M. A. Atieh, F. Rahman, T. Laoui, R. Karnik, and E. N. Wang, “Nanostructured materials for water desalination,” Nanotechnology, vol. 22, no. 29, p. 292001, Jun. 2011. [↩]