Engineering Energy Level Alignment in Lead Sulfide Quantum- Dot Photovoltaics through Ligand Exchange
- Category: Energy, Nanotechnology
- Tags: patrick brown, vladimir bulovic
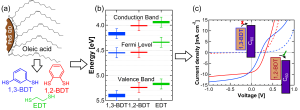
Figure 1: (a) Schematic depiction of oleic-acid-coated lead sulfide QDs (PbsS QDs); the oleic acid ligands are exchanged for 1,3-benzenedithiol (1,3-BDT), 1,2-benzenedithiol (1,2-BDT), or ethanedithiol (EDT). (b) Valence band, Fermi level, and conduction band energies determined from ultraviolet photoelectron spectroscopy and absorption spectroscopy for PbS QDs treated with different ligands. (c) Current-voltage curve of PbS QD/C60 donor-acceptor heterojunction photovoltaics, where the PbS QDs are ligand-exchanged with 1,3-BDT (blue curve) or 1,2-BDT (red curve). The insets depict the expected mode of device operation.
Solar cells based on lead sulfide colloidal quantum dots (PbS QDs) have made dramatic improvements in efficiency in recent years, owing in large part to novel surface passivation techniques involving organic or inorganic ligands. In spite of these advances, the influence of these ligand treatments on QD charge transport, doping, and energy level structure is still incompletely understood. Different ligand treatments have been shown to alter the valence band and conduction band binding energies in InAs and CdSe QDs by up to 0.3 eV through modification of QD-ligand interface dipoles[1][2][3], but the importance of similar energy shifts for electronically relevant ligands in PbS QD solids and the effects of these shifts on photovoltaic device performance have hitherto been unexplored. Through a combination of ultraviolet photoelectron spectroscopy and carrier transport measurements, we demonstrate that even between structurally and chemically similar ligands such as 1,2-ethanedithiol (EDT), 1,2-benzenedithiol (1,2-BDT), and 1,3-benzenedithiol (1,3-BDT) (Figure 1a), sizeable changes in the PbS QD valence band energy and work function can dramatically affect photovoltaic device performance. In particular, 1,2-BDT-treated PbS QDs are found to have a ~ 0.2 eV shallower valence band energy and work function than 1,3-BDT-treated PbS QDs (Figure 1b); this shift necessitates a re-optimization of the energy levels of the electron- and hole-extracting contacts for solar cells employing these ligands (Figure 1c). In addition, 1,2-BDT and 1,3-BDT treatments result in higher power conversion efficiencies than EDT treatment for PbS Schottky junction and ZnO/PbS heterojunction photovoltaics, respectively, despite the fact that BDT-treated films demonstrate a lower charge carrier mobility than EDT-treated films. These results emphasize the importance of optimizing interfacial energy offsets and surface passivation, rather than solely the carrier mobility, in PbS QD photovoltaics.
- M. Soreni-Harari, N. Yaacobi-Gross, D. Steiner, A. Aharoni, U. Banin, O. Millo, and N. Tessler, “Tuning energetic levels in nanocrystal quantum dots through surface manipulations,” Nano Letters, vol. 8, pp. 678-684, Jan. 2008. [↩]
- A. M. Munro, B. Zacher, A. Graham, and N. R. Armstrong, “Photoemission spectroscopy of tethered CdSe nanocrystals: Shifts in ionization potential and local vacuum level as a function of nanocrystal capping ligand,” ACS Applied Materials and Interface, vol. 2, pp. 863-869, Mar. 3010. [↩]
- J. Jasieniak, M. Califano, and S. E. Watkins, “Size-dependent valence and conduction band-edge energies of semiconductor nanocrystals,” ACS Nano, vol. 5, pp. 5888-5902, June 2011. [↩]