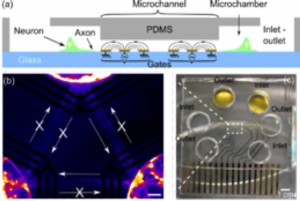
Electrokinetic Control of Axonal Growth
- Category: MEMS & BioMEMS
- Tags: joel voldman, thibault honegger
Dynamic control of axonal outgrowth holds the potential to establishing a range of in-vivo and in-vitro applications including perpiheral nerve section injury recovery, neural computers, and neural interfaces. Although many axon guidance clues like surface topography[1], biochemical[2] or external forces[3] have been investigated, those methods do not provide downscaling and dynamic control of axonal growth.
We have introduced the use of AC electrokinetics to dynamically control axonal growth in cultured rat hippocampal neurons. We find that the application of modest voltages at frequencies on the order of 105 Hz can cause developing axons to be stopped adjacent to the electrodes while axons away from the electric fields exhibit uninhibited growth. By switching electrodes on or off, we can reversibly inhibit or permit axon passage across the electrodes. We make use of our dynamic control over axon elongation to create an axon-diode via an axon-lock system that consists of a pair of electrode “gates” that either permit or prevent axons from passing through as shown in Figure 1a-c. Finally, we developed a neural circuit consisting of three populations of neurons, separated by three axon-locks to demonstrate the assembly of a functional, engineered neural network as shown in Figure 1b. Action potential recordings demonstrate that the AC electrokinetic effect does not harm axons, and Ca2+ imaging demonstrated the unidirectional nature of the synaptic connections. AC electrokinetic confinement of axonal growth has potential for creating functional, configurable, and directional neural networks[4].
We have extended this work to demonstrate the control of axonal growth in collagen scaffold. The scaffold is confined in a microfluidic channel of three different heights where axons can develop over electrodes. When the channel height was limited to the size range of the growth cone (~ 3 mm), axon repulsion in a 2D plane was observed. We find that developing axons in the microchannel are repelled from the electrodes and follow the field lines until lower field strength (Figure 2a). Axons that grow in the same channel but further away from the electrodes show uninhibited growth. When the channel height is in the range of the growth cone (~10 mm), axonal growth is slowed down by the AC field. Finally, when the channel height is significantly bigger than the growth cone (~ 50 mm), axons develop in 3D into the scaffold and are repelled from the electrodes in the depth of the scaffold itself, where the minimum plane height is linked to the magnitude of the electric field as presented in Figure 2a-b.
This new technology provides a powerful tool to confine axonal growth and leads the way to dynamic configurable neuronal networks.
- Figure 1: (a) Side-view schematic of axon diode, (b) Fluorescent false-colored image of a 12-DIV neuronal network configured by the axon-lock system. The plain arrows represent the direction of the diode. (c) Images of axon diode chip showing three microchambers and electrodes.
- Figure 2: (a) Picture of developing axons in a 3-μm-high channel filled with collagen after 4 DIV. The axons are repelled and guided by the activated electrodes. (b) Confocal microscope reconstruction of developing axons in a 50-μm-high channel. The axons develop in a parallel plane above the activated electrodes. (c) Quantification of the minimum height of this plane for several voltages.
- J. W. Lee, K. S. Lee, N. Cho, B. K. Ju, K. B. Lee, and S.-H. Lee, “Topographical guidance of mouse neuronal cell on SiO2 microtracks,” Sensors and Actuators B 128, 252-257, 2007. [↩]
- A. M. McCormick and N. D. Leipzig, “Neural Regenerative Strategies Incorporating Biomolecular Axon Guidance Signals,” An. Biomed Eng, 2012. [↩]
- T. Wu, T. A. Nieminen, S. Mohanty, J. Miotke, R. L. Meyer, H. Rubinsztein-Dunlop, and M. W. Berns, “A photon-driven micromotor can direct nerve fibre growth”. Nat Photon, pp. 62-67, 2012. [↩]
- T. Honegger, M. A. Scott, and J. Voldman, Lab on a Chip, 13, 4, pp. 589-598, 2013 [↩]