Continuous RBC Removal Using Spiral Channel with Trapezoidal Cross-section
- Category: MEMS & BioMEMS
- Tags: jongyoon han, lidan wu
Red blood cells (RBC) are the most abundant cell component in many biological fluids, including blood, bone marrow aspirate, and peritoneal aspirate. Depletion of contaminating RBCs from those samples is often an indispensable sample preparation step before the application of any clinical and diagnostic tests[1], while avoiding artificial alteration on the phenotypes of sorted cells is an important criterion for all studies. The achievement of minimal artifact is especially important in the case of removing RBCs from human blood to isolate white blood cells (WBCs), which play a key role in carrying out and mediating the immune response to various pathogens. The information extracted from the isolated WBCs would be meaningful to facilitate disease prognosis only when the key features of WBCs’ original state are not masked by the sample preparation artifacts. However, several cases have been reported that the conventional methodologies for blood cell separation on the macroscale, including differential centrifugation and selective erythrocyte lysis, could result in altered imuno-phenotype[2] or impaired viability[3] of the isolated WBCs. Meanwhile, passive continuous microfluidic separation techniques utilizing the size-dependent hydrodynamic effects[4][5][6] have been considered as an alternative approach to bypass the issues associated with macroscale blood cell separation methods.
In this work, we improved the separation resolution of curvilinear microchannel while maintaining the high-throughput feature by modifying the channel cross-section to be trapezoidal rather than rectangular and demonstrated its ability for efficient RBC depletion from human blood sample with negligible effect on polymorphonuclear leukocyte (PMN) immune-phenotype as compared to selective erythrocyte lysis method[7]. To our knowledge, this is the first experimental demonstration where the asymmetry velocity field within a trapezoid spiral channel affects the inertial focusing phenomenon, indicating the feasibility of using channel cross-sectional geometry (other than width and depth) as a parameter for optimization of a curvilinear inertial microfluidic sorter.
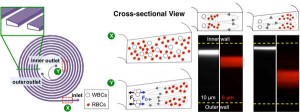
- Figure 1: Design principle of developed size-based sorter for RBC removal. Spiral channel with trapezoid cross-section (500 μm x 70~ 100 μm, W x H) has higher separation resolution than spiral channel with rectangular cross-section (500 μm x 90 μm) of similar dimension. Sample collected from inner outlet of developed trapezoid cross-sectional spiral would be enriched for WBCs.
- Figure 2: Device performance on blood sample. (A) Single pass recovery percentage of blood components for sample collected from inner outlet at different hematocrits. (B) Comparison of PMN activation by spiral and other RBC removal techniques, including density centrifugation (MP-RM) and RBC lysis, based on flow cytometry analysis of CD66b+ CD18+ cells.
- W. G. Guder, S. Narayanan, H. Wisser, and B. Zawra, Samples: From the Patient to the Laboratory: The impact of preanalytical variables on the quality of laboratory results, 1st ed. Darmstadt, Germany: GIT Verlag, 1996. [↩]
- J. Lundahl, G. Halldén, M. Hallgren, C. M. Sköld, and J. Hed, “Altered expression of CD11b/CD18 and CD62L on human monocytes after cell preparation procedures,” Journal of Immunological Methods, vol. 180, pp. 93-100, 1995. [↩]
- C. J. van Oss, P. M. Bronson, E. A. Dinolfo, and K. C. Chadha, “Two methods for the removal of erythrocytes from buffy coats for the production of human leukocyte interferon,” Immunological Communications, vol. 10, pp. 549-555, 1981. [↩]
- H. W. Hou, A. A. S. Bhagat, W. C. Lee, S. Huang, J. Han, and C. T. Lim, “Microfluidic Devices for Blood Fractionation,” Micromachines, vol. 2, pp. 319-343, 2011. [↩]
- L. R. Huang, E. C. Cox, R. H. Austin, and J. C. Sturm, “Continuous Particle Separation Through Deterministic Lateral Displacement,” Science, vol. 304, pp. 987-990, May 14, 2004. [↩]
- M. Yamada, M. Nakashima, and M. Seki, “Pinched Flow Fractionation: Continuous Size Separation of Particles Utilizing a Laminar Flow Profile in a Pinched Microchannel,” Analytical Chemistry, vol. 76, pp. 5465-5471, Sep. 1, 2004. [↩]
- L. Wu, G. Guan, H. W. Hou, A. A. S. Bhagat, and J. Han, “Separation of Leukocytes from Blood Using Spiral Channel with Trapezoid Cross-section,” Analytical Chemistry, vol. 84, pp. 9324-9331, Nov. 6, 2012. [↩]