Condensation on Micro/Nanostructured Superhydrophobic Surfaces
- Category: Energy, Materials, Nanotechnology
- Tags: evelyn wang, nenad milijkovic
Water condensation on surfaces is a ubiquitous phase-change process that plays a crucial role in nature and across a range of industrial applications including energy production, desalination, and environmental control. Nanotechnology has created opportunities to manipulate this process through the precise control of surface structure and chemistry, thus enabling the biomimicry of natural surfaces, such as the leaves of certain plant species, to realize superhydrophobic condensation. However, this “bottom-up” wetting process is inadequately described using typical global thermodynamic analyses and remains poorly understood. In this work, we study condensation on well-defined structured surfaces spanning a wide range of length scales from 100 nm to 10 μm and functionalized using several hydrophobic thin films (Figure 1). We found that the emergent morphology of isolated droplets interacting with the surface structures during growth is primarily defined by the pinning behavior of the local contact line within the structures[1]. Depending on the relationship between the structure length-scale and the droplet nucleation density, the dominant condensed droplet morphology can switch to one that is thermodynamically unfavorable in a global sense due to local contact line de-pinning. We show how these isolated condensed droplet morphologies arise by quantitatively describing growth in terms of characteristic local energy barriers and extend this view to explain the role of droplet-droplet interactions in determining emergent droplet morphology (Figure 2). This result contrasts the common macroscopic view of wetting behavior for individual droplets. The understanding of droplet growth behavior developed provides a rational basis to develop optimized condensing surfaces. This mechanistic framework also has implications for understanding condensation behavior on nature’s superhydrophobic surfaces and ice formation on structured surfaces that initiates with the condensation of sub-cooled water.

Figure 1: Scanning electron micrographs of fabricated pillar geometries. (a) Electrodeposited Au nanopillars defined by an anodic alumina template. (b) Si nanopillars fabricated using interference lithography and metal-assisted wet etching. (c) Si nanopillars fabricated using e-beam written mask and DRIE. (d) Si micropillars fabricated using optical lithography and DRIE.
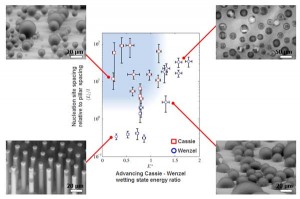
Figure 2: Regime map characterizing the dominant wetting behavior observed during condensation with coordinates of 〈L〉/l and E*, where 〈L〉 is the average spacing between droplet nucleation sites, l is the pillar-to-pillar pacing, E^*=-1/(r∙cosθ_a), r=1+πdh/l^2, d is the pillar diameter, h is the pillar height, and θa is the smooth surface advancing contact angle. Cassie morphologies emerge at large 〈L〉/l and E^*≤1 (shaded region). Wenzel morphologies emerge at low 〈L〉/l and/or E^*≳1.
- R. Enright, N. Miljkovic, A, Al-Obeidi, C. V. Thompson, and E. N. Wang, “Superhydrophobic Condensation: The role of energy barriers and size scale,” Langmuir, 2012, vol. 28, no. 40, p. 14424–14432. [↩]