Cell Pairing for Studying Immunity
- Category: MEMS & BioMEMS
- Tags: burak dura, joel voldman
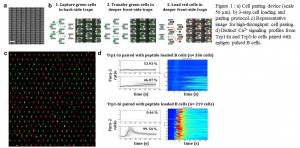
Figure 1 a) Cell pairing device (scale 50 µm). b) 3-step cell pairing protocol. c) High-throughput cell pairing. d) Distinct Ca2+ dynamics from Trp1-hi and Trp1-lo cells paired with peptide loaded B cells.
Cell-cell interactions are crucial for proper functioning of the immune system because direct cell-cell contacts largely govern the successful progression of adaptive immune responses. The heterogeneity inherent in these interactions plays a critical role in the functional outcome produced. Assessing the heterogeneity in the initial activation and connecting it with the endpoint function would, therefore, clarify the signaling cascades involved in the observed outcomes. Current methods to study this heterogeneity are mainly limited by the control over pairing, and thus by initiation of activation and low throughput. To remedy this situation, we developed a high-throughput microfluidic cell-pairing platform for studying the activation kinetics of immune cells. We adapted the microfluidic device from a previously developed chip for studying cell reprogramming[1] and altered device design and geometries to accommodate much smaller immune cells. The device comprises a dense array of weir-based hydrodynamic cell traps that contain a back-side single-cell trap and front-side two-cell trap (Figure 1a). Using a 3-step loading protocol (Figure 1b), we achieved 67 ± 12 % (n=18) pairing efficiencies with a range of 40-86 %, the highest ever reported for such smaller cells (Figure 1c). We used our microfluidic platform to dissect the activation kinetics of T cells from two lines of trans-nuclear mice, Trp1-hi and Trp1-lo, which recognize the identical peptide-MHC complex with markedly different affinities yet are equivalent in their ability to curtail the growth of B16 melanoma in vivo. We paired Trp1-hi/lo T cells with antigen-loaded B cells in a highly parallel and synchronous manner and measured the activation profiles through Ca2+ imaging. Our results show inherent heterogeneity within the clonal population of each cell type and indicate significant differences in the activation dynamics and activation percentages between the two lines (Figure 1d). These findings emphasize how immune cells dissociate the affinity of interaction and heterogeneity in the initial activation from a robust in vivo protective ability. Study of activation cascades in this well-defined system provides substantial insight into how variation leads to robust functional outcomes in immunity.
- A. M. Skelley, O. Kirak, H. Suh, R. Jaenisch, and J. Voldman, “Microfluidic control of cell pairing and fusion,” Nature Methods vol. 6, pp. 147-152, 2009. [↩]