Cell-based Sensors for Measuring Impact of Microsystems on Cell Physiology
- Category: Energy
- Tags: anna fendyur, joel voldman
The use of microsystems to manipulate and study cells in microenvironments is continually increasing. However, along with such increase in usage comes a growing concern regarding the impact of these microsystems on cell physiology. In this project, we are developing a set of cell-based fluorescent sensors to measure the impact of common stresses experienced by cells in microsystems.
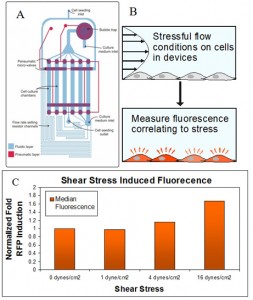
The shear-stress sensor is the most relevant sensor to the microfluidic and bio-MEMS community and the most challenging sensor to create, since relevant molecular pathways have not been entirely elucidated. To address this quastion, we characterized the gene expression profile of NIH3T3 cells using a multi-flow microfluidic device (Figure 1a) that can simultaneously apply a logarithmic (1000×) range of shear stress conditions[1]. Using qRT-PCR we identified the genetic node sensitive to shear and designed a red fluorescence protein (RFP) reporter plasmid. We transfected and cloned this plasmid into NIH3T3s to create our shear stress response sensor. We seeded this sensor in the same device, exposed it to flow for 0.1-3 hours, and assayed for fluorescence expression changes using flow cytometry (Figure 1b). Using our sensor, we were able to successfully correlate device shear conditions to population level inductions (Figure 1c).
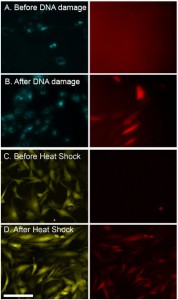
In parallel we are developing a sensor that responds to activation of the p53 protein pathway, for generalized DNA damage analysis. The DNA damage sensor couples RFP expression to activation of p53. The new sensor will be identified by constitutive cyan (mTurquoise) color (Figure 2a-b).
Each cell-based fluorescent sensor will respond to one particular stress agent. The number of sensors can be combined for multiplexed analysis of multiple stresses at once, as might occur in a typical microsystem. Each sensor will use different constitutive colors to indicate the type of sensor and RFP as the activation color. For this purpose we re-engineered our existing heat shock sensor[2][3] to express constitutive yellow (YPET) and red (RFP) as the activation color (Figure 2c-d).
- Figure 1: (A) Multi-flow microfluidic device, showing the fluidic layer (blue) where the cells are grown and the pneumatic layer (red) containing microvalves. (B) Approach to quantify shear stress using our sensor. (C) Correlation of fluorescence induction to shear stress intensity.
- Figure 2: NIH3T3 cells. (A) DNA damage sensor before and (B) 24 hrs after DNA damage with methyl methanesulfonate – DNA alkylating agent. The left images – constitutive cyan; right – red fluorescent DNA damage reporter. (C) Heat shock sensor before and (D) 16 hrs after a heat shock ( 43oC) for 60 min. Left images are constitutive YPET reporter; right images are red fluorescent heat shock reporter. Scale: 100 μm.
- Y.-C. Toh and J. Voldman, “Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate [↩]
- S. P. Desai and J. Voldman, “Cell-based sensors for quantifying the physiological impact of microsystems,” Integrative Biology, vol. 3, pp. 48-56, 2011. [↩]
- S. P. Desai and J. Voldman, “Measuring the impact of dielectrophoresis on cell physiology using a high-content screening platform,” in Micro Total Analysis Systems ’08, San Diego, CA, USA, 2008, pp. 1308-1310. [↩]