A Novel Microfluidic “Cell-based” Blood Dialysis Platform for Septic Murine Model
- Category: MEMS & BioMEMS
- Tags: han wei hou, jongyoon han
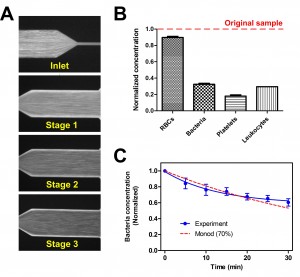
Sepsis is an adverse systemic inflammatory response caused by microbial infection in blood. We have reported a microfluidic approach for removal of microbes and inflammatory cellular components from whole blood, inspired by the in vivo phenomenon of leukocyte margination[1],[2]. We also developed a multiplexed blood filtration platform to demonstrate the bacteria removal capability in vivo using a septic mouse model (Figure 1). As blood flows through the margination channel, deformable red blood cells migrate to the axial center (Fahreaus effect), resulting in margination of other cell types towards the sides. Bacteria-depleted blood is collected at the center outlet and returned directly to the animal, as in a complete dialysis circuit. In vitro experiments using human blood spiked with FITC-conjugated Escherichia coli (E. coli) indicated a bacteria removal efficiency of ~70%; inflammatory cellular components (platelets and leukocytes) were also depleted by >70% (Figure 2). To mimic in vivo mouse filtration, a blood sample (~1mL, similar to mouse blood volume) spiked with fluorescent E. coli was subjected to continuous filtration in a closed loop circuit using a peristaltic pump. Experimental data obtained were in good agreement with the Monod kinetics model, achieving ~40% decrease in bacteria concentration after 30 minutes of filtration. The developed technique offers significant advantages: high throughput (~2mL/hr) and label-free separation for non-specific removal of blood-borne pathogens. The device is ideal for the mouse model: the filtration flow rate (90 mL/kg/hr) is comparable to high-volume hemofiltration (45-60 mL/kg/hr) used for humans in clinical settings. Unlike current extracorporeal blood purifications which mostly focus on cytokines removal, we hypothesize that a broad spectrum removal of bacteria and inflammatory cellular components (platelets and leukocytes) could help modulate the host inflammatory response as a blood cleansing method for sepsis treatment.
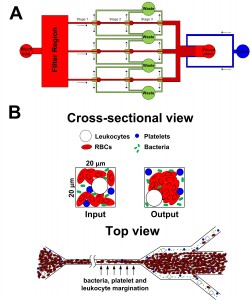
- Figure 1: Schematic illustration of the (A) 3-channel margination microdevice and (B) separation principle at each bifurcation stage. Deformable RBCs migrate axially to the channel center, resulting in margination of other cell types (bacteria, platelets and leukocytes) towards the channel sidewalls while the center outlet collects the bacteria-depleted blood.
- Figure 2: (A) Average composite images illustrating margination of FITC-labelled E. coli at each bifurcation stage. (B) Histogram showing the normalized concentration of different cellular components at the filtered centre outlet. (C) Plot indicating the decrease in bacteria concentration over time for 1mL of blood sample at 30 µLmin-1.
- H. W. Hou, H. Y. Gan, A. A. S. Bhagat, L. D. Li, C. T. Lim, and J. Han, “A microfluidics approach towards high-throughput pathogen removal from blood using margination,” Biomicrofluidics, vol. 6, pp. 024115-024113, 2012. [↩]
- H. L. Goldsmith and S. Spain, “Margination of leukocytes in blood flow through small tubes ” Microvascular Research, vol. 27, pp. 204-222, 1984. [↩]