A Microfluidic ‘‘Baby Machine’’ for Cell Synchronization
- Category: MEMS & BioMEMS
- Tags: scott manalis
Common techniques used to synchronize eukaryotic cells in the cell cycle often impose metabolic stress on the cells or physically select for size rather than age. To address these deficiencies, a minimally perturbing method known as the ‘‘baby machine’’ was developed previously. In the technique, suspension cells are attached to a membrane, and as the cells divide, the newborn cells are eluted to produce a synchronous population of cells in the G1 phase of the cell cycle. However, the existing ‘‘baby machine’’ is suitable only for cells that can be chemically attached to a surface. Here, we present a microfluidic ‘‘baby machine’’ in which cells are held onto a surface by pressure differences rather than chemical attachment (Figures 1 and 2). As a result, our method can in principle be used to synchronize a variety of cell types, including cells that may have weak or unknown surface attachment chemistries. We validate our microfluidic ‘‘baby machine’’ by using it to produce a synchronous population of newborn L1210 mouse lymphocytic leukemia cells in G1 phase[1].
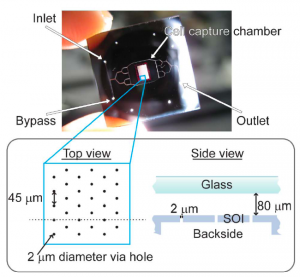
- Figure 1: Image of the synchronization device illuminated from the backside, showing branching fluidic channels that lead into a cell capture chamber with 2109 2-mm diameter holes onto which cells can be loaded. In the schematic diagram, the side view illustrates the cross section along the dotted line in the top view. The channels are etched into the top glass wafer, and the 2-mm holes and fluidic ports are etched into the bottom SOI wafer.
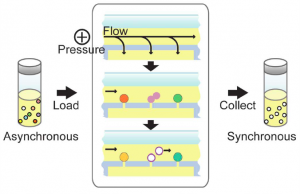
- Figure 2: Operation of the device is controlled entirely by pressure differences. A positive pressure applied at the inlet creates both a pressure differential in the vertical direction across the holes to capture cells and a pressure drop in the horizontal direction to generate lateral flow for the collection of daughter cells. An asynchronous population of cells is fed into the device and captured into the holes; with a constant slow perfusion of cell culture medium, the cells grow within the device. As they divide, the newborn cells, which are not anchored to trapping holes, are carried away in the fluid flow and eluted into a collection chamber. The eluted cells then compose a synchronous population of cells that has just completed cytokinesis and entered the G1 phase of the cell division cycle.
- J. Shaw, K, Payer, S. Son, W. H. Grover and S. R. Manalis, “A microfluidic ‘‘baby machine’’ for cell synchronization,” Lab on a Chip, vol. 12, pp. 2656-2663, 2012. [↩]