Studying Autocrine Signaling for Growth in Tumor and Stem Cells
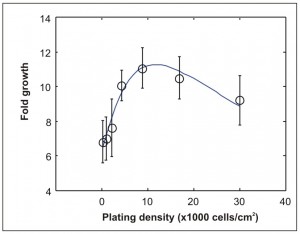
Figure 1: Measured fold growth versus density for 2-day culture of mouse embryonic stem cells (circles). The solid line shows a fit from our model.
Autocrine signaling plays a key role in tumorigenesis and in the maintenance of various physiologic states. Our research involves the use of cell-patterning techniques to investigate the role of autocrine signaling during in vitro expansion of embryonic stem cells and cancer cells.
Expanding on our previous experimental work, recently we have also developed numerical models of autocrine signaling. Cells act as sources for autocrine factors. However, cells also possess receptors that the factors can bind to. Finally, in addition to protein transport, uptake of nutrients and production of metabolites are also important processes to account for, especially for longer culture periods. We have previously found an optimal density for 2-day culture of mouse embryonic stem cells. Our models suggest that the positive feedback on growth provided by autocrine signaling combined with the negative feedback provided by nutrient depletion can account for the presence of such an optimal density (Figure 1).
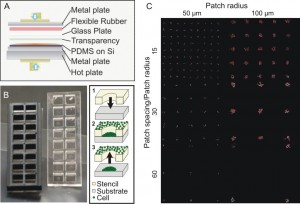
Figure 2: Platform to study autocrine spatial operation. (A) Stencils were fabricated by uniformly pressurizing uncured PDMS on master silicon wafer that contains prefabricated microposts. (B) Finished stencils with a plastic gasket to separate cultivation of different patterns. To pattern cells, a stencil is attached to substrate and loaded with cell suspension. After allowing the cells attach, the stencil is peeled off. The gasket is then placed on top of the cultivation slide. (C) Resulting patterns of tumor cells with different patterning geometries.
For our study of autocrine signaling in tumor cells, we continued to investigate the role of autocrine signaling on heterogeneity in tumor growth. Using A431 epidermoid carcinoma cells as our model, we used stencil-cell patterning to position cells as square-latticed arrays of circular cell patches of varying size and spacing (Figure 2). Unlike randomly-plated cell culture where cells experience differing amounts of cell-cell contacts and irregular intercellular spacing, our platform ensures the direct modulation of autocrine signaling while keeping other contributing signals intact. We are investigating the use of the developed platform as a novel tool to quantify the spatial operation of autocrine signaling. Existing methods require prior knowledge of the underlying autocrine loops and therefore cannot be applied to less characterized biological systems. Our technique will be useful for in vitro investigation of cancer therapeutics and enables the systematic modulation of autocrine-promoted growth.