Stencil-and-Flip Cell Patterning for Generation of Dual Stem Cell Microenvironments
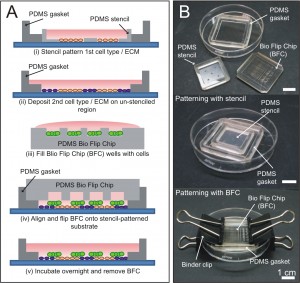
Figure 1: Stencil-and-Flip Cell Patterning (SAF-CP) for presenting dual microenvironments to a stem cell colony. (A) Schematic on the operations of SAF-CP. (B) Images showing components and assembly of SAF-CP.
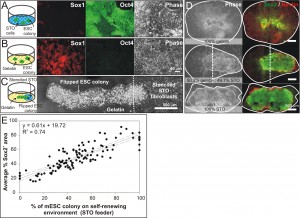
Embryonic development is a complex dynamic process, whereby spatial organization of molecular signals instructs stem cells to adopt various differentiation fates at different locations [1]. Recapitulating this process in vitro will greatly facilitate the mechanistic understanding of embryonic development. However, current in vitro models are unable to reproduce the developmental environment adequately. To realize the aim of building a complex developmental model, we developed the Stencil-and-Flip Cell Patterning (SAF-CP) technique to present multiple spatially organized microenvironments to a single population of stem cells [2]. SAF-CP combines two established patterning technologies i.e., stencil and Bio Flip Chip (BFC) [3] but uses them in a sequentially aligned format to create spatially organized stem cell microenvironments in vitro (Figure 1). To validate that spatial organization of microenvironments translates to differential instruction of stem cell fates, we used SAF-CP to selectively present self-renewing (STO fibroblast feeder + N2B27 medium) (Figure 2A) and neuronal differentiating (gelatin + N2B27 medium) (Figure 2B) microenvironments to a single mouse embryonic stem cell (mESC) colony. (Figure 2C) After one week of culture, we observed that self-renewal (Sox2) and neuronal precursor (Nestin) markers had a spatial distribution coinciding with the patterned dual microenvironments (Figure 2D). The ratio of self-renewal-to-neuronal phenotypes within a colony was observed to be dependent on the relative extent of the two microenvironments presented to the colony (quantifiable by the percentage colony area on STO and gelatin, respectively) (Figure 2E). Our results demonstrate the utility of the SAF-CP in building in vitro models that recapitulate the organizational-instructive traits of stem cell niches, allowing us to emulate stem cell development more realistically.
Figure 2: Self-renewing and neuronal differentiating dual microenvironments direct reciprocal fates in a mouse embryonic stem cell (mESC) colony. (A) mESCs cultured on STO fibroblasts in N2B27 medium self-renew as indicated by Oct4. (B) mESCs cultured on gelatin in N2B27 medium differentiated into neuronal precursors as indicated by Sox1. (C) SAF-CP presented both self-renewing and differentiating environments to a single mESC colony. (D) Expression of self-renewing (Sox2) and neuronal (Nestin) markers after one week of culture. Colonies presented with dual microenvironments exhibited intermediate phenotypes compared to those presented with single microenvironment. (E) Average Sox2 expression in mESC colonies presented with varying ratios of self-renewing to differentiating microenvironments.
References
- J. Rossant and P. Tam, “Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse,” Development, vol. 136, pp. 701-713, 2009. [↩]
- Y.C. Toh, J. Voldman, “In vitro construction of complex stem cell microenvironments by Stencil-and-Flip Cell Patterning” The 13th International Conference on Miniaturized Systems for Chemistry and Life Sciences (μTAS), Nov. 2009. [↩]
- A. Rosenthal, A. MacDonald, J. Voldman, “Cell Patterning Chip for Controlling the Stem Cell Microenvironment” Biomaterials, vol. 28, pp. 3208-3216, July 2007. [↩]