Monitoring Cell Physiology in Microbioreactors
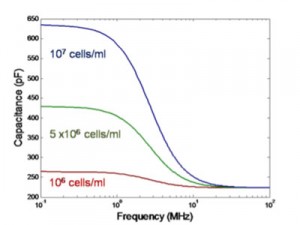
Figure 1: Modelled dielectric spectroscopy of CHO cells. As the live cell density increases, the ΔC reading increases linearly.
Mammalian cell culture dominates the biopharmaceutical industry for biotherapeutics including monoclonal antibodies, vaccines, and growth factors. However, mammalian cells are also the most sensitive to changes in the culture environment, e.g., mechanical agitation, nutrient depletion, and waste byproduct accumulation. Hence, maintaining cell viability in mammalian cell cultures for an extended period of time is an important limiting factor for mammalian cell cultures [1]. Currently, state-of-the-art micro-bioreactors estimate cell density by measuring the turbidity of the culture using an Optical Density (OD) sensor [2]. Unfortunately, OD sensors measure light scattered by cells which may not be accurate due to cell aggregation and the influence of cell shape. Additionally, biomass measurements do not discriminate between live and dead cells. An online sensor that explicitly measures viable cells in a micro-bioreactor is necessary.
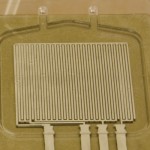
Figure 2: Picture of device made by sputtering platinum electrodes on a milled piece of polycarbonate, which forms the bottom half of the dielectric spectroscopy test chip.
Dielectric spectroscopy is a promising online sensor for cell viability in micro-bioreactors [3]. The difference between the low-frequency and high-frequency capacitance measurements (ΔC = CLF – CHF) in the radio frequency regime gives the capacitance contributed by the cells to the total capacitance of the suspension. Due to the fact that most dead cells no longer have an intact membrane, defined as a membrane that is selectively impermeable to ions in the solution, they do not contribute to the capacitance reading (ΔC). By calibrating the measured capacitance with cell suspensions of known cell densities, the number of live cells in the culture can be determined, shown in Figure 1. When dielectric spectroscopy is combined with OD measurements, the percent cell viability can be utilized to optimize the yield of a mammalian cell culture in a micro-bioreactor. The first part of our project involves designing and calibrating a dielectric spectroscopy sensor for a micro-scale bioreactor, shown in Figure 2.
References
- J. Goswami, A.J. Sinskey, H. Stellar, G.N. Stephanopaulos, and D.I.C. Wang, “Apoptosis in batch cultures of Chinese hamster ovary cells,” Biotechnology and Bioengineering, vol. 62, no. 6, pp. 632-640, Mar. 1999. [↩]
- H.L.T. Lee, P. Bocazzi, R.J. Ram, and A.J. Sinskey, “Microbioreactorarrawys with integrated mixers and fluid injectors for high-throughput experimentation with pH and dissolved oxygen control,” Lab On A Chip, vol. 6, pp 1229-1235, July 2006. [↩]
- K. Asami, “Characterization of biological cells by dielectric spectroscopy,” Journal of Non-Crystalline Solids, vol. 305, pp. 268-277, 2002. [↩]