Microscale Controlled Continuous Cell Culture
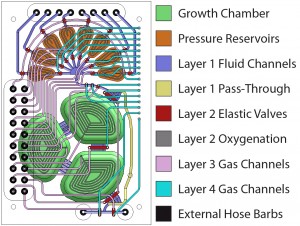
Figure 1: Schematic of the micro-continuous culture device. Eight inputs lines provide control over the chemical composition of the medium within the growth reservoir. Fluids are introduced through a peristaltic pump, which injects liquid into the growth chamber.
For systems biology, the models are more often limited by the absence of reliable experimental data than by available computational resources. Unfortunately, there is still great difficulty in making the leap from genetic and biochemical analysis to accurate verification with conventional culture growth experiments due to variations in culture conditions. Measurements of metabolic activity through substrate and product interactions or cellular activity through fluorescent interactions are highly dependent on environmental conditions and cellular metabolic state. For such experiments to be feasible, continuous cultures [1] [2] utilizing control strategies must be developed to measure chemical concentrations, introduce chemical inputs, and remove waste. An integrated microreactor system with built-in fluid metering will enable environmental control and programmable experiments capable of generating reproducible data.
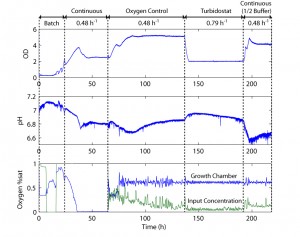
Figure 2: Plot of the cell density, pH, oxygen concentration, and oxygen control over time for growth on defined glucose medium. The chip is run in multiple modes, demonstrating the measurement and control capabilities of the microfluidic system.
The chip shown in Figure 1 is fabricated out of a rigid plastic, polycarbonate, utilizing PDMS membranes for actuation and pumping [3] The fabrication process for bonding plastic-PDMS hybrid devices has been described previously [4]. Mixing and oxygen delivery is performed through membranes between the fluidic and actuation layers of the growth chamber sections. Chip reliability is demonstrated over a 2-week culture where multiple steady states are reached. Culture experiments are performed with E. coli ATCC31883 in 5 g/L glucose minimum-salts defined medium supplemented with 100 ug/ml ampicillin. Cell density is measured through forward-scattering with an optical sensor at 585 nm in a path length of 0.8 mm. Multiple operation modes are shown in Figure 2 including batch, fed batch, oxystat, chemostat, and turbidostat. Full control is demonstrated under turbidostatic steady state, where the cell density is closed-loop-controlled at a cell density of 2, by dynamically varying the flow rate. Turbidostatic steady state also allows the extraction of the cell maximum growth rate of 0.79 h-1 in minimum salts medium.
References
- J. Monod, “La technique de culture continue, theorie et applications,”Annales de I’Institut Pasteur (Paris), 79, pp. 390-410 (1950). [↩]
- A. Novick and L. Szilard, “Description of the chemostat,” Science, 112, pp. 715-716 (1950). [↩]
- V. Studer, G. Hang, A. Pandolfi, M. Ortiz, W. F. Anderson, and S. R. Quake, “Scaling properties of a low-actuation pressure microfluidic valve,” J. Applied Physics, 95, pp. 393-398 (2004). [↩]
- K. S. Lee and R. J. Ram, “Plastic-PDMS bonding for high pressure hydrolytically stable active microfluidics,” Lab-on-a-Chip, 9, pp. 1618-1624 (2009). [↩]