Microfluidic Studies of Cancer Invasion: New High-throughput Assays to Study Microenvironmental Effects
Microfluidics offer a unique platform for screening the effects of the biochemical and biophysical microenvironment on cellular phenotype, while allowing for increasing the experimental throughput compared to traditional assays. In this work we study the effect of interstitial flow on tumor cell migration and the interactions between tumor and endothelial cells during tumor cell entry into a blood vessel (intravasation) in a 3D extracellular matrix. Based upon previous work over the past years in our lab [1] [2], we recently developed a new design with a 10-fold increase in the number of matrix regions allowing for increased data collection capabilities. The design consists of independently addressable microfluidic channels interconnected through 3D matrices, wherein tumor and endothelial cells can be seeded, while establishing interstitial flow and/or chemoattractant gradients through the matrix. We have also developed a protocol for applying fluid-flow induced shear stress on the endothelial monolayer on the channel for studying its effects on tumor cell intravasation. After applying a shear stress of 3 dynes/cm2 for 24 hours, we observed alignment of the endothelial cells in the flow direction consistent with previous studies. Using live cell imaging and in the absence of any directional microenvironmental cues, we observed both a quiescent and sprouting endothelium, while some tumor cells invaded in 3D randomly and others reached towards the endothelial monolayer.
Tumor cells exposed to interstitial flow preferentially migrated along streamlines, and the relative percentage of cells migrating upstream and downstream was a function of chemokine receptor activity and intercellular distance. At low seeding densities, cells preferentially migrated upstream. However, at high intercellular distances, cells preferentially migrated downstream but reverted to upstream migration with blocking of a single cell receptor, CCR7. These data provide supporting evidence to the autologous chemotaxis model and suggest that a competing paracrine pathway provides a stimulus for upstream migration.
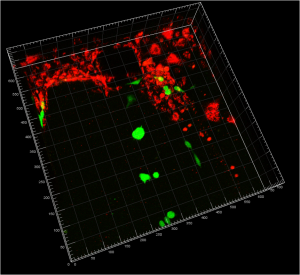
- Figure 1: Confocal reconstruction of endothelial cells (red) on the channel and tumor cells (green) seeded inside the collagen gel.
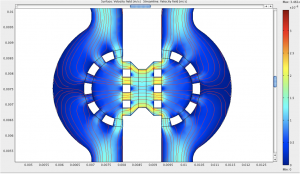
- Figure 2: Flow streamlines and interstitial flow magnitude in a device designed to study the effect of interstitial flow on tumor cell migration.
References
- S. Chung, R. Sudo, P.J. Mack, C.R. Wan, V. Vickerman, and R.D. Kamm , “Cell migration into scaffolds under co-culture conditions in a microfluidic platform,” Lab Chip, vol. 9: pp. 269-75, January 2009. [↩]
- V. Vickerman, J. Blundo, S. Chung, and R.D. Kamm, “Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging,” Lab on a Chip, vol. 8: pp. 1468-77, September 2008. [↩]