Microfluidic Perfusion for Modulating Stem Cell Diffusible Signaling
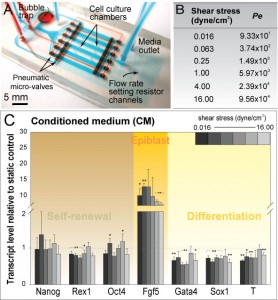
Figure 1: (A) Microfluidic perfusion array for probing shear stress effects on mESCs. (B) Range of shear stress used in this study and corresponding Peclet number (Pe). (C) Gene profile for a range of applied shear values under conditions of saturated soluble environment (CM).
Stem cell phenotype and function are influenced by microenvironmental cues comprised of the cell-cell, cell-extracellular matrix (ECM), cell-media interactions, and mechanical forces. Our research focuses on developing microscale systems for controlling the cellular microenvironment of mouse embryonic stem cells (mESCs), in particular mechanical forces (i.e., shear stress) and cell-media interactions (i.e., diffusible signaling).
The use of embryonic stem cells (ESCs) for clinical therapeutic applications requires expansion of the pluripotent cells in bioreactors, where the cells are subjected to fluid shear stresses [1]. In comparison to macroscale bioreactors, microfluidic perfusion systems allow for study of shear stress effects on mESCs across a wide range of flow-rates. We demonstrate the utilization of a multiplex microfluidic perfusion device as a powerful experimental tool to probe shear stress effects in a graded, quantitative manner, allowing us to identify a shear-modulated marker, Fgf5 (Figure 1A-C). Such approach facilitates further targeting of molecular players implicated in shear stress mechanotransduction.
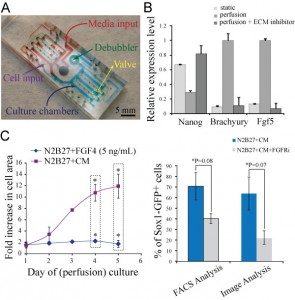
Figure 2: (A) Microfluidic perfusion device used in this study. (B) Gene expression levels of Nanog (self-renewal marker), Brachyury, and Fgf5 (early differentiation markers) for different culture conditions: standard culture in a dish (self-renewal) and perfusion culture (self-renewal & self-renewal with small molecule inhibitor of ECM signaling). (C) Growth analysis of mESC perfusion cultures in defined differentiation medium (N2B27) supplemented with Fgf4 and soluble factors saturated medium (N2B27+CM) (left). Sox1 (differentiation marker) expression for saturated medium (N2B27+CM), and in the presence of an inhibitor of Fgf signaling (right).
Flow properties at the microscale enable fine-tuning of soluble factors transport, which plays a crucial role in determining ESC fate. To modulate cell-media interactions in mESCs self-renewal and differentiation, we utilized a two-layer microfluidic perfusion device allowing for parallel comparison of different cell culture conditions (Figure 2A) [2]. Our results demonstrate that mESCs under conditions of reduced autocrine signaling in self-renewing culture conditions express decreased levels of self-renewal markers and at the same time upregulated levels of early differentiation markers. In contrast, an interruption of cell-ECM interactions yields opposite results (Figure 2B). Similarly, we demonstrated that mESCs failed to proliferate under conditions of minimized autocrine signaling during neuronal differentiation. In addition, we found that autocrine Fgf4 signaling is implicated as a crucial factor in acquiring neuronal identity of mESCs, while alone it cannot restore the growth of mESCs undergoing differentiation (Figure 2C).
References
- Steiner D. et al., 2010, Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension, Nature Biotechnology, vol. 28, pp. 361-364, Mar. 2010. [↩]
- K. Blagović, L.Y. Kim, A.M. Skelley, and J. Voldman, “Microfluidic control of stem cell diffusible signaling,” in Proc. of Twelfth International Conference on Miniaturized Systems for Chemistry and Life Sciences, San Diego, CA, pp. 677-679, Oct. 2008. [↩]