Microfluidic Control of Cell Pairing and Fusion
Cell fusion has been used for many purposes, including generating hybridomas and reprogramming somatic cells. The fusion step is the key event in initiating these procedures. Standard fusion techniques, however, provide poor and random cell contact, leading to low yields. While different approaches can be used successfully for reprogramming, the cell lines generated are not yet suitable for potential therapeutic applications in humans, and many questions remain about the process of nuclear reprogramming. A more efficient cell-pairing and fusion method could answer these questions. Skelley et al. have therefore developed a microfluidic device to trap and properly pair thousands of cells [1] (Figures 1 and 2). Using this device, the pairing efficiency for different cell types, including fibroblasts, mouse embryonic stem cells and myeloma cells, is as high as 70%. The device is compatible with both chemical and electrical fusion protocols, with membrane reorganization efficiencies of up to 89%. These properties render the device particularly suitable for our current study of the underlying mechanism of fusion-based reprogramming.
Having established the basis for high-yield cell-pairing measurements using stem cells, we are developing a similar device for the statistical, kinetic study of immune cell populations. Immune responses are largely mediated by cell-cell interactions. In particular, natural killer cells and cytotoxic T cells form conjugates with pathogenic and cancer cells in order to fight disease Errors in these and other immune cell-cell interactions can lead to fatal immune diseases. The study of these intricate cell-cell interactions at the molecular scale is crucial for understanding the dynamics and specificity of the immune response. Conventional techniques, such as bulk measurements or immobilization of cell pairs on a dish, preclude gathering of sufficient data on single cell pairs for meaningful statistics of cell-cell interactions. We propose to overcome the limitations of traditional methods by visualizing and controlling the pairing of thousands of individual immune cell pairs simultaneously. Primary mouse lymphocytes (~6 μm diameter), a common model system in immunology, are significantly smaller than stem cells (~18 μm diameter), making a device redesign necessary. We have employed a numerical, finite- element fluid- dynamic model as a guide to optimize pairing efficiencies by altering geometric properties (Figure 3). Our device is furthermore compatible with standard staining methods, such as antibody staining and ratiometric calcium flux measurements. The next step will be to fabricate the new devices and evaluate their pairing efficiency.
-
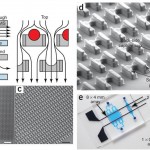
Figure 1 [1]: (a) Schematic of the microfluidic device operation and structure. The flow passes under the cell trap, directing the cells into the capture cup. The support pillars maintain the proper vertical gap. (b) Scanning electron micrograph image of the 2 mm x 2 mm device, which contains 1,166 cell traps. (c) Magnified image of the device, showing the densely packed structures. (d) Detail of the trap structure, including the larger front-side and smaller back-side capture cups (14 µm tall, 18µ m wide, 25–40 µm deep, and 10 µm wide 5 µm deep, respectively), along with support pillars (7.5µ m wide x 35–50 µm long x 6–8 µm tall). (e) Different arrays that can pair and capture thousands of cells in parallel. Adapted from [1] .
- Figure 2 [1]: (a) Cells are first loaded ‘up’ toward the smaller back-side capture cup. (b) The direction of the flow is reversed, and the cells are transferred ‘down’ into the larger front-side capture cup two rows below. (c) The second cell type is loaded from the top, and cells are captured in front of the first cell type. (d) Red and green fluorescence image overlay image of 3T3s loaded into the device. Adapted from [1] .
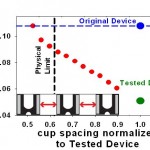
- Figure 3: To redesign the cell-pairing device for immune cells, we have established a finite-element fluids model. The plot shows the predicted effect of reducing cup spacing (normalized by the downscaled immune cell device) on the relative flow rate through the capture cups. The blue line represents the original cell- fusion device. The green line represents simple downscaling of the original device, yielding low flow and, presumably, pairing efficiency. The physical limit (black line) indicates the smallest cup spacing possible that still permits cells to pass between two cups. The model shows that reducing the horizontal cup spacing can increase flow through the cups and ultimately pairing efficiencies for immune cells.
References