Iso-dielectric Separation of Cells and Particles
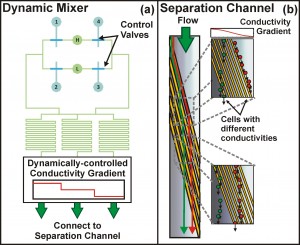
Figure 1: Iso-dielectric separation with dynamic conductivity control. (a) We have developed a valve scheme that enables real-time control of the electrical conductivity gradient through time-multiplexed sampling of high (‘H’) and low (‘L’) conductivity inlets. Here, complementary sets of valves, (1, 4) and (2, 3), are actuated to dynamically control the conductivity profile entering the separation channel, illustrated in (b).
The development of new techniques to separate and characterize cells with high throughput has been essential to many of the advances in biology and biotechnology over the past few decades. Continuing or improving upon this trend – for example, by developing new avenues for performing genetic and phenotypic screens – requires continued advancements in cell-sorting technologies. Towards this end, we are developing a novel method for the simultaneous separation and characterization of cells based upon their electrical properties. This method, iso-dielectric separation (IDS), uses dielectrophoresis (DEP, the force on a polarizable object [1]) and a medium with spatially varying conductivity to sort electrically distinct cells while measuring their effective conductivity. It is similar to iso-electric focusing, except that it uses DEP instead of electrophoresis to concentrate cells and particles to the region in a conductivity gradient where their polarization charge vanishes [2] [3].
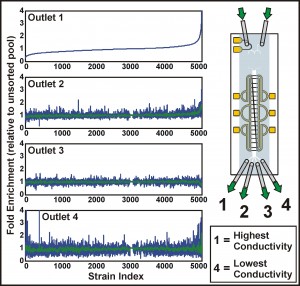
Figure 2: Screening the yeast deletion strains for distinct electrical phenotype. The plots show the fold enrichment of each of ~5000 deletion strains, collected from different outlets, relative to the unsorted pool. Strains are ordered according to their enrichment in the highest conductivity fraction (outlet 1); for outlets 2 through 4, green curves give a moving average of the enrichments.
Previously, we have demonstrated the ability to perform continuous-flow, label-free, non-binary separations using IDS on a wide variety of cells and particles, while simultaneously extracting quantitative information from these samples as they are sorted [4]. We are currently focusing on extending these capabilities to perform genome-wide characterizations of electrical properties in the budding yeast Saccharomyces cerevisiae. The most recent implementation of the device uses a valve scheme that enables real-time control of the conductivity gradient, along with the ability to rapidly switch samples for sorting and characterization (Figure 1). These developments increase the throughput of the device, making the systematic characterization of both pooled and unpooled cell libraries feasible. To date, we have applied IDS to a pooled screen of the yeast deletion collection, identifying several genes associated with distinct electrical properties (Figure 2). The improved understanding of the relationship between a cell’s genotype and its physical properties enabled by IDS suggests its potential as a new high-content screening platform.
References
- H.A. Pohl and J.S. Crane, “Dielectrophoresis of cells,” Biophysical Journal, vol. 11, pp. 711-727, 1971. [↩]
- M.D. Vahey and J. Voldman, “An Equilibrium Method for Continuous-Flow Cell Sorting Using Dielectrophoresis,” Analytical Chemistry, vol. 80, no. 9, pp. 3135-3143, 2008. [↩]
- M.D. Vahey and J. Voldman, “Iso-dielectric Separation: A new method for the continuous-flow screening of cells,” Micro Total Analysis Systems ’06, vol. 2, pp. 1058-1060, 2006. [↩]
- M.D. Vahey and J. Voldman, “High-Throughput Cell and Particle Characterization Using Isodielectric Separation,” Analytical Chemistry, vol. 81, no. 7, pp. 2446-2455, 2009 [↩]