Increase of Sensitivity of ELISA Using a Multiplexed Electrokinetic Preconcentrator
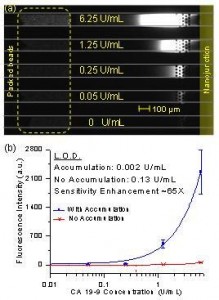
Figure 1: (a) Experimental image of concentration-enhanced bead-based ELISA of CA 19-9 in serum. (b) Dose-response curve of CA 19-9 ELISA. Sensitivity enhancement due to product accumulation was ~ 65 fold.
Enzyme-Linked Immunosobent Assay (ELISA) is the gold standard in biodetection due to its high reproducibility and sensitivity. Still, the ultimate detection sensitivity of ELISA is not good enough to tackle the challenges of biomarker detection. This led to the development of many novel ultra-high-sensitivity immunoassay platforms. However, additional sensitivity comes with the cost of added complexity in amplification chemistry or complicated devices. Being able to measure analytes at concentrations below the current detection limits, without modifying the basic workflow of ELISA, would be very useful.
We utilized a nanofluidic preconcentration device to continuously accumulate enzymatic product molecules from ELISA into a much smaller volume and increase its local concentration significantly, therefore achieving a lower limit of detection. An important advantage of this scheme is that the enzymatic product being concentrated is the same, and thus the same device setup is applicable regardless of the antigen. We also demonstrated multiplexing capability by assaying five samples simultaneously in a single device.
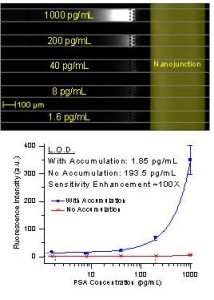
Figure 2: (a) Experimental image of concentration-enhanced ELISA of PSA in serum. (b) Dose-response curve of PSA ELISA. Sensitivity enhancement due to product accumulation was ~ 100 fold.
We showed enhanced detection sensitivity in two cases: 1) when the enzymatic reaction is on-chip and 2) when the enzymatic reaction is off-chip. In the first case, immunobinding was performed using beads as a solid phase, and the beads were physically trapped in a microfluidic channel. When fresh substrate solution was flown through the bead pack, it was enzymatically converted into fluorescence product and accumulated downstream. In the second case, the complete ELISA workflow, including substrate conversion, was performed in a 96-well plate. After that, the converted molecules were injected into the device and electrokinetically concentrated. Using the first method, we demonstrated a 65-fold lower limit of detection for CA 19-9, a human pancreatic cancer marker, in control serum. The detection limit for human prostate specific antigen (PSA) in donkey serum was 100 times lower using the second method.
References
- K. Sato, M, Tokeshi, T. Odake, et. al., “Integration of an immunosorbent assay system: analysis of secretory human immunoglobulin A on polystyrene beads in a microchip,” Anal. Chem., vol. 72, no. 6, pp. 1144-1147, Feb. 2000.
- Y.C. Wang and J.Y. Han, “Pre-binding dynamic range and sensitivity enhancement for immune-sensors using nanofluidic preconcentrator,” Lab Chip, vol. 8, pp. 392-394, 2008.
- L.F. Cheow, S.H. Ko, S.J. Kim, K.H. Kang, and J. Han, “Increasing the sensitivity of enzyme-linked immunosorbent assay using multiplexed assay using multiplexed electrokinetic concentrator,” Anal. Chem., vol. 82, no. 8, pp. 3383-3388, Mar. 2010.