Free-flow Zone Electrophoresis of Peptides and Proteins in PDMS Microchip for Narrow Isoelectric-Point (pI) Range Sample Prefractionation Coupled with Mass Spectrometry
Isoelectric point (pI)-based fractionation is ideally suited for the first-dimensional separation because the pI value of any peptide or protein can be simply estimated from the sequence information. Therefore, the pI-based fractionation techniques can be highly specific to target peptides and proteins. Due to this benefit, there have been previous efforts to integrate isoelectric focusing (IEF) into mass spectrometry (MS)-based proteome analysis processes [1] [2] [3] [4]. While the physical coupling between capillary IEF and ESI-MS is straightforward, the buffer systems for IEF separation (carrier ampholytes, a complex mixture of amphoteric small molecules) have low compatibility with electrospray (ESI)-MS interfaces. In view of this current deficit, we have developed an ampholyte-free, two-step cascaded microfluidic sorting technique based on free-flow zone electrophoresis that isolates the molecules of interest from a small sample volume of 100 mL within a narrow and freely adjustable pI range (£ 1 pH units), even below pH 3 and beyond pH 10 [5]. To create a salt bridge for free-flow electrophoresis in PDMS chips, we printed a submicron-thick hydrophobic layer on a glass substrate and created an electrical junction gap for free-flow zone electrophoresis. With this sorting device, as shown in Figure 1, we demonstrated binary sorting of peptides and proteins in standard buffer systems and validated the sorting result with liquid chromatography (LC)/MS. In Figure 2, the sorting result of the acidic peptides < pH 7 is shown as an example. Furthermore, we coupled two sorting steps via off-chip titration and isolated peptides within specific pI ranges from sample mixtures, where the pI range was simply set by the pH values of the buffer solutions. This pI-based binary sorting device, with its simplicity of fabrication and a sorting resolution of 0.5 pH unit, can potentially be a high-throughput sample fractionation tool for targeted proteomics using LC/MS.
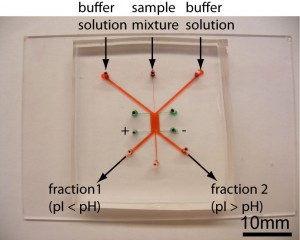
- Figure 1: Free-Flow Zone Electrophoresis (FFZE) device in PDMS micro chip format. The sample is injected from the autosampler into the middle inlet. The width can be varied by adjusting the flow rates of the sheath buffer solutions. The separated sample fractions are collected from the three outlet channels.
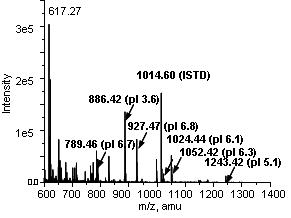
- Figure 2: ESI-MS result of a single fractionation of 8 peptides (40 nM) at pH 7.0 with E=279V/cm and 0.5μL/min. From a mixture of 8 acidic and basic peptides, we could successfully fractionate acidic peptides with pIs of 3.6, 5.1, 6.1, 6.3, 6.7 and 6.8.
References
- L. Yang, C. S. Lee, S. A. Hofstadler, and R. D. Smith, “Characterization of microdialysis acidification for capillary isoelectric focusing- microelectrospray ionization mass spectrometry,” Analytical Chemistry, vol. 70, no. 23, pp. 4945-4950, Oct. 1998. [↩]
- D. Kohlheyer, G. Besselink, S. Schlautmann, and R. B. Schasfoort, “Free-flow zone electrophoresis and isoelectric focusing using a microfabricated glass device with ion permeable membranes,” Lab on a Chip, vol. 6, pp. 374-380, Jan. 2006. [↩]
- D. Kohlheyer, J. C. Eijkel, J. C., S. Schlautmann, A. van den Berg, and B. M. Schasfoort, “Microfluidic high-resolution free-flow isoelectric focusing,” Analytical Chemistry, vol. 79, no. 21, pp. 8190-8198, Sep. 2007. [↩]
- B. Fonslow and M. Bowser, “Optimizing band width and resolution in micro-free flow electrophoresis,” Analytical Chemistry, vol. 78, no. 24, pp. 5369-5374, Nov. 2006. [↩]
- Y.-A. Song, M. Chan, C. Celio, S. Tannenbaum, J. Wishnok, and J. Han, “Free-flow zone electrophoresis of peptides and proteins in PDMS microchip for narrow pI range sample prefractionation coupled with mass spectrometry,” Analytical Chemistry, vol. 82, no. 6, pp. 2317-2325, Feb. 2010. [↩]