Formation of a Cobalt-based Oxidation Catalyst from Thin-film Cobalt Anodes

Figure 1: (left) The thin-film cobalt metal electrode is shown with electrochemical masking lacquer (red middle section) and copper tape with conductive epoxy. (right) The thin-film cobalt electrode is shown during operation during catalytic activity. Bubbles forming on the surface of the electrode are molecular oxygen.
Splitting water into molecular oxygen and molecular hydrogen is a compelling strategy for clean energy storage [1]. In order to achieve efficient conversion from water to oxygen or hydrogen fuels, catalytic materials are necessary, and their integration into technological devices is critical. We are currently working with a water-oxidation catalyst that is capable of assisting the transformation from water to molecular oxygen [2]. We report progress towards integration of the catalyst with a thin-film metal electrode surface that may facilitate its utilization in devices.
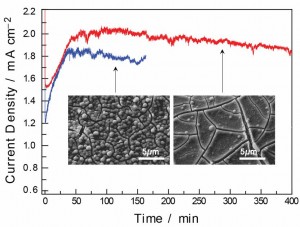
Figure 2: Current density traces for bulk electrolysis at 1.1 V (vs Ag/AgCl) in 0.1M potassium phosphate, pH 7, for (—) catalyst formation on FTO-coated glass anodes with 0.5 mM Co2+ and (—) catalyst formation on thin-film cobalt anodes. Both electrodes demonstrate comparable competence in current passage throughout the bulk electrolysis. SEM images at 5000 times magnification of the Co-Pi film formed on each electrode are shown.
Eight-hundred-nanometer thin films of cobalt metal are sputter-deposited on a non-conductive glass substrate to serve as electrode scaffolds for formation of the cobalt-based water-oxidation catalyst. The middle one-third of the electrode is masked with an electrochemical masking lacquer positioned at the air-water interface during electrode operation. The catalyst is formed by electrolytic deposition in neutral aqueous solutions of 0.1 M potassium phosphate where the thin films of cobalt serve as the conductive electrodes as well as the source of Co2+ cations for formation of the catalyst material. The formation of catalyst films on the thin-film cobalt electrodes is compared to similar films formed from ionic solutions of Co2+ on fluorinated tin oxide (FTO) conductive glass electrodes, reported previously [2]. We report that the chemical composition and the current densities achieved during operation in the catalytic regime are similar for catalytic films on thin-film cobalt and FTO electrodes. The surface morphology of the catalyst film on the thin-film cobalt electrodes is smoother than those found for films formed from Co2+ solutions onto FTO electrodes.
This work demonstrates that thin-film cobalt metal electrodes are effective for cobalt-based water-oxidation catalyst formation and successfully eliminate the need for utilizing solutions of Co2+ in formation of the catalyst films. Further development of the cobalt electrode is in progress with the goal of increasing the catalytic current output of the catalyst films formed directly on thin-film metal electrodes.
References
- N. S. Lewis and D. G. Nocera, “Powering the planet: Chemical challenges in solar energy utilization,” Proc. Natl. Acad. Sci. U.S.A. vol. 103, pp 15729-15735, 2006. [↩]
- M. W. Kanan and D. G. Nocera, “In situ formation of an oxyen-evolving catalyst in neutral water containing phosphate and Co2,” Science vol. 321, pp 1072-1075, 2008. [↩] [↩]