Flow-through Electric Double-layer Capacitor for Water Desalination
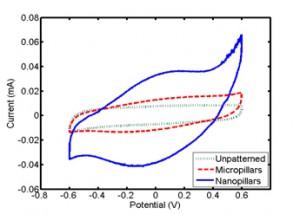
Figure 1: Cyclic voltammograms of the silicon-based materials. Each test used a 1 M NaCl electrolyte with a sweep rate of 50 mV/s. The silicon nanopillars exhibited the largest capacitance due to the 13-fold increase in surface area.
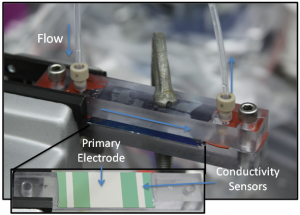
Figure 2: Image of the flow setup. The primary adsorption material is located in the center with platinum conductivity sensors at the inlet and the outlet.
In capacitive deionization (CDI), salt water is passed through two polarized electrodes, whereby the salt is adsorbed onto the electrode surface and removed from the water stream. This approach has received renewed interest for water desalination due to the development of new high surface area carbon-based nanomaterials [1] [2] [3] [4] [5] [6]. However, there is currently limited understanding as to how electrode geometry, surface properties, and capacitance affect ion capture. In this work, we experimentally investigate various standard carbon-based electrode materials, including activated carbon and carbon cloths, as well as microfabricated silicon structures for CDI. The silicon nanopillars were fabricated using 50-nm polystyrene beads as a physical mask on silicon oxide and etched using reactive ion etching. Electrochemical characterization through cyclic voltammetry was used to determine the electrochemical properties of each material (Figure 1). In addition, a mini-channel test cell was fabricated to perform parametric studies on ion capture. The flow cell can incorporate various electrode materials while monitoring the real-time solution conductivity at the outlet to determine the amount of salt removed from the solution (Figure 2). By controlling electrode geometry and chemistry in these studies, the work helps elucidate transport mechanisms and provide insight into the design of optimal materials for capacitive deionization.
References
- J. C. Farmer, et al., “Capacitive deionization of NaCl and NaNO3 solutions with carbon aerogel electrodes,” Journal of the Electrochemical Society, vol. 143, pp. 159-169, Jan. 1996. [↩]
- Y. Gao, et al., “Electrosorption behavior of cations with carbon nanotubes and carbon nanofibres composite film electrodes,” Thin Solid Films, vol. 517, pp. 1616-1619, Jan. 1 2009. [↩]
- H. B. Li, et al., “Electrosorption behavior of graphene in NaCl solutions,” Journal of Materials Chemistry, vol. 19, pp. 6773-6779, 2009. [↩]
- L. X. Li, et al., “Ordered mesoporous carbons synthesized by a modified sol-gel process for electrosorptive removal of sodium chloride,” Carbon, vol. 47, pp. 775-781, Mar. 2009. [↩]
- H. J. Oh, et al., “Nanoporous activated carbon cloth for capacitive deionization of aqueous solution,” Thin Solid Films, vol. 515, pp. 220-225, Sep. 25 2006. [↩]
- M. W. Ryoo, et al., “Role of titania incorporated on activated carbon cloth for capacitive deionization of NaCl solution,” Journal Of Colloid And Interface Science, vol. 264, pp. 414-419, Aug. 15 2003. [↩]