Electrochemical Development of Sub-10-nm-pitch Hydrogen Silsesquioxane (HSQ) Structures in Saline Solution

Figure 1: SEM images comparing electrochemical development with salty development of 9-nm-pitch (a) and 12-nm-pitch (b) nested-L structures patterned in ~15-nm-thick HSQ on silicon. Experiments demonstrated comparable resolution of electrochemical development and salty development of HSQ and no obvious influence of the substrate (silicon and silicon coated with a thin chrome layer) on the development quality.
A new method for developing hydrogen silsesquioxane (HSQ), a negative electron-beam resist with demonstrated 9-nm-pitch resolution [1], is presented, avoiding the addition of hydroxyl ions to the developer. This method addresses and possibly overcomes physical and chemical factors influencing the achievable resolution of HSQ during development [2].
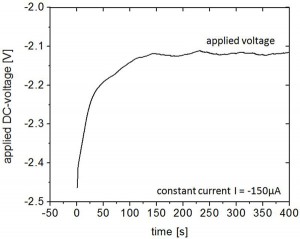
Figure 2: Time evolution of applied potential between a sample with chrome coating and saline solution during electrochemical development for a constant current of I = -150μA. At the beginning, the HSQ resist acted as high-resistive layer on the sample surface, and thus a larger potential had to be applied. With proceeding development, the applied potential decreased as more and more chrome surface was exposed until the resist was removed from the complete surface and a steady current value was reached.
The development mechanism of the acidic-like resist HSQ in basic solution is assumed to be the conversion of HSQ into negatively charged, water-soluble ions under the generation of hydrogen, simplified as: ≡Si‒H + OH– → ≡Si‒O– + H2 [3]. Thereby the Si-H bound of the HSQ is transformed into a Si-O– configuration that is chemically more favorable for the basic pH value of the developer solution. But the transition of Si‒H to Si‒OH and even into Si‒O– also occurs in water under normal circumstances, although with slim probability. We enhance this reaction by applying a potential between sample and solution, thereby enforcing an electrical current.
We have demonstrated the development of HSQ in non-alkaline saline solution (4% NaCl in DI-water) and applied potential of about 2.5V and achieved comparable resolution to salty development (1% NaOH and 4% NaCl in DI-water) with pattern dimensions as good as 9-nm-pitch nested-L structures, see Figure 1 and 2. Development was observed only at the negative electrode and with applied potential. Therefore we hypothesize that the unexposed resist is either directly reduced to negatively charged HSQ ions by the transfer of electrons or water is electrolyzed and thereby developer is locally produced on the resist.
Electrochemical development of resist opens a new pathway for development that is not limited by diffusion and possible charging of the sample surface. Furthermore, the negatively charged resist ions are repelled by the substrate. The speed and quantity of development can be adjusted by the applied potential.
References
- J.K.W. Yang, B. Cord, K.K. Berggren, J. Klingfus, SW. Nam, KB. Kim, and M.J. Rooks, “Understanding of hydrogen silsesquioxane electron resist for sub-5-nm-half-pitch lithography,” Journal of Vacuum Science & Technology B, vol. 27, no. 6, pp. 2622-2627, 2009. [↩]
- B. Cord, J. Yang, H.G. Duan, D.C. Joy, J. Klingfus, and K.K. Berggren, “Limiting factors in sub-10 nm scanning-electron-beam lithography,” Journal of Vacuum Science & Technology B, vol. 27, no. 6, pp. 2616-2621, 2009. [↩]
- J. Kim, W.L. Chao, B. Griedel, XG. Liang, M. Lewis, D. Hilken, and D. Olynick, “Understanding the base development mechanism of hydrogen silsesquioxane,” Journal of Vacuum Science & Technology B, vol. 27, no. 6, pp. 2628-2634, Nov., 2009. [↩]