CMP Slurry Abrasive Particle Agglomeration Modeling
In this work we propose a particle-agglomeration model for CMP, to understand the creation and behavior of agglomerated slurry abrasive particles arising during the CMP process. Slurry agglomeration is a major cause of defectivity, as well as poor consumable utility due to sedimentation [1] [2] [3]. Our approach goes beyond prior slurry abrasive-particle-modeling attempts, which have focused on qualitative correlations [4], empirically assumed relationships of those correlations on particle size distributions [5], or quantitative correlations of purely mechanistic behavior of agglomerated particles, without considering chemical effects [2] [6].
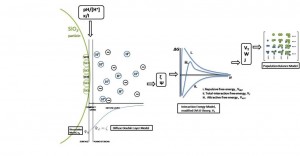
Our proposed model will provide both a qualitative and quantitative description of agglomeration in slurry abrasive particles during CMP that includes the chemical kinetic and mechanistic behaviors of both the slurry abrasive particles and the slurry electrolyte, enabling more accurate process control, increased consumable utility, and possible reduction of defectivity. The framework for this new agglomeration model is shown in Figure 1. The proposed model treats the slurry composition as a colloidal suspension of charged colloidal silica in an electrically neutral aqueous electrolyte. First, a theoretical relationship between the measurable chemical parameters of the slurry’s aqueous electrolyte, the surface potential of the abrasive particles, and corresponding zeta potential between the agglomerated abrasive particles is developed. Secondly, this zeta potential is used in a modified DVLO-interaction-potential model to determine the particle-interaction potentials due to both the attractive van Der Waals forces and repulsive electrostatic interactions. Finally, the total interaction potential created is then used to define a stability ratio for slow versus fast agglomeration and corresponding agglomeration rate equations between particles; these are used in a discrete population balance framework to describe the final particle size distribution with respect to time and agglomerate composition. Current work is focused on experimental development, validation, and extension of the theoretical model.
References
- F.-C. Chang, S. Tanawade, and R. K. Singh, J. Electrochem. Soc., 156, H39 (2009). [↩]
- R. Biswas, Y. Han, P. Karra, P. Sherman and A. Chandra, J. Electrochem. Soc., 155, D534-D537 (2008). [↩] [↩]
- M. Moinpour, A. Tregub, A. Oehler, and K. Cadien, Mater. Res. Bulletin, 766, (2002). [↩]
- S. Ramarajan, Y. Li, M. Hariharaputhiran, Y.-S. Her, and S. V. Babu, Electrochem. Solid-State Lett., 3(5), 232-234 (2000). [↩]
- A. R. Mazaheri and G. Ahmadi, and J. Electrochem. Soc., 150, G233-G239 (2003). [↩]
- F.-C. Chang and R. K. Singh, and J. Electrochem. Solid-State Lett., 12, H127-H130 (2009). [↩]