Top-down Templating of Protein Assembly: Complex Molecular Self-assembly Routes to Biological Device Fabrication
- Category: Nanotechnology
- Tags: jae-byum chang, karl berggren, yong ho kim
Templated assembly of biomolecules can create complex nanostructured devices with precisely tailored chemical or biological responses, with applications in, for example, nanoscale patterning for electronics, biomedical devices, or environmental sensors. In this research project, we focus on developing methods for creating complex molecular top-down templating of assembled structures of protein that will be relevant to a range of devices.
We examined a range of EM staining techniques for cortexillin, which is coiled-coil protein and forms a parallel homodimer as an actin-binding domain. The protein constructs have the addition of single cysteine residues at either the N- or C- terminus that would facilitate binding gold metal surfaces, as Figure 1(A) shows. Weakly staining the rod shape of proteins in uranyl acetate resulted in observation of structures 20 nm in length and 4~5 nm in width, which roughly matches the expected protein model from crystallographic structure, as in Figure 1(B). We also tested tagging of the cortexillin homodimer using gold nanoparticles, which resulted in gold points colocalized to the proteins. This provides a straightforward way to visualize single protein molecules using TEM and SEM.
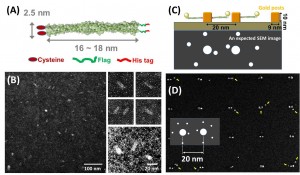
Figure 1: (A) The structure of cortexillin (B) TEM images of cortexillin stained with 2% uranyl acetate and inset images (right) showing morphology of protein such as fat rods in higher magnification. (C) Single molecular protein array in the project, presents gold tagging to His-tag in protein and Cys residue facilitates binding to gold posts. (D) SEM images of protein array in the gold post pattern with 20-nm pitch.
We are also been developing a method to position proteins into the patterned surfaces with sub-10-nm resolution. We have functionalized an e-beam patterned surface with gold, resulting in two-post arrays with a pitch varying from 10 to 50 nm. The cysteine of the cortexillin homodimer can then be used to direct the attachment of the protein to the posts via thiol-gold attachment chemistry. The cortexillin proteins also contain a C-terminal hexa-histidine tag, which allows for the attachment of Ni-NTA gold nanoparticles (NTA: nitrilotriacetic acid) via the coordination of the charged histidines around the nickel ion (Kd ≅ 10-6 M). Figure 1(C) illustrates the assembly scheme of single protein array on a gold post pattern, where the cysteine residue in a coiled-coil protein that allows binding to surfaces of gold posts and the hexa-histidine sequence allows attachment of Ni-NTA gold nanoparticles. By performing multiple step protocol of: e-beam patterning of poly methyl methacrylate (PMMA) resist, gold post generation on PMMA pattern, PMMA development, cleaning by Plasma etching, and incubation in the Cys-modified protein solution, we achieved results suggesting the formation of single-molecule protein arrays around the gold posts of the pattern as observed by SEM. SEM imaging of the pattern after incubation with protein reveals that there is a relatively low density of protein binding, as indicated by small gold nanoparticles around the gold post patterns, as Figure 1(D) shows. Here, we could develop the method using electron-beam lithography to create a gold post pattern with sub-10-nm resolution pitch close to biomolecular scale, which could then be used to template novel systems including DNA, protein, and other biomolecules.
- D. Klinov, K. Atlasov, A. Kotyar, B. Dwir, and Kapon E., “DNA nanopositioning and alignment by electron-beam-induced surface chemical patterning,” Nano Letters, vol. 7, p. 3583, 2007.
- M. R. Diehl, K. Zhang, H. J. Lee, and D. A. Tirrell, “Engineering cooperativity in biomotor-protein assemblies,” Science, vol. 311, p. 1468, 2006.
- N. Zizlsperger, V. N. Malashkevich, S. Pillay, and A. E. Keating, “Analysis of coiled-coil interactions between core proteins of the spindle pole body,” Biochemistry, vol. 47, p. 11858, 2008.