Microfluidic Perfusion for Modulating Stem Cell Diffusible Signaling
Stem cell phenotype and function are influenced by microenvironmental cues that include cell-cell, cell-extracellular matrix (ECM), and cell-media interactions (i.e., diffusible signaling), which we can control using microscale systems. Our research focuses on cell-ECM and cell-media control of mouse embryonic stem cells (mESCs). Cells are constantly secreting and responding to soluble signals, the removal of which can be mediated by modulating flow properties at the microscale [1] . To assess the contribution of cell-secreted factors to mESC differentiation and self-renewal, we utilized a two-layer microfluidic perfusion device allowing for parallel comparison of different cell culture conditions (see Figure 1A).
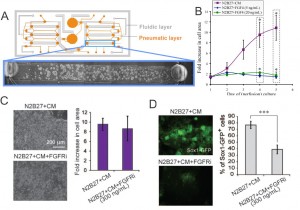
Our results demonstrate that mESCs do not grow in differentiation conditions with minimal autocrine signaling, even with supplementation by Fgf4, a signal that has been shown to be a crucial factor in differentiation toward a neuronal stem cell fate, while they do grow when supplemented with media saturated with soluble signals (conditioned media, CM) (see Figure 1B). Consistent with this effect, inhibiting the Fgf4 receptor does not affect growth of mESCs in differentiation conditions (as Figure 1C shows), but it does affect differentiation toward a neuronal stem cell fate (as in Figure 1D) [2] .
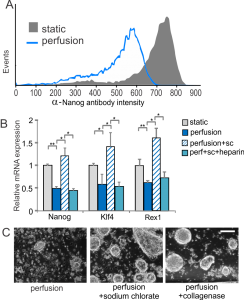
ESCs grown under self-renewal conditions are able to proliferate without conditioned media, but they lose expression of the self-renewal marker Nanog (see Figure 2A), results that, together with signaling and downstream differentiation assays, indicate differentiation towards an epiblast-like state under conditions that had previously been shown to be sufficient for self-renewal. This differentiation can be reversed by disrupting the ECM using sodium chlorate, which affects the ability of growth factors to bind to the ECM (as Figure 2B shows). This effect is evident based on colony morphology and can be duplicated by disrupting the matrix using collagenase (see Figure 2C) [3] . Together, these results indicate the importance of diffusible cell-secreted signals for mESC growth and ECM-based signals for mESC self-renewal.
- Figure 1: (A) Schematic of microfluidic perfusion device and mESCs growing in a single chamber. (B) Growth analysis of perfusion cultures in differentiation medium (N2B27) supplemented with Fgf4, and soluble factor-saturated medium (N2B27+CM). (C) Images of cells cultured under perfusion in the indicated conditions and corresponding growth analysis. (D) Fluorescent images of cells grown under perfusion in the indicated conditions and corresponding Sox1-GFP+ cell frequency assessed by flow cytometry. * = p<0.05; *** = p<0.001.
- Figure 2: (A) Flow cytometry histogram of Nanog protein levels from cells grown in self-renewal media. (B) Expression levels of self-renewal markers in the presence and absence of sodium chlorate with or without the addition of soluble heparin in self-renewal media. (C) Representative images of cells grown under perfusion in self-renewal media with or without sodium chlorate or collagenase. Scale bar = 200 µm.
- L. Przybyla and J. Voldman, “Probing embryonic stem cell autocrine and paracrine signaling using microfluidics,” Annual Review of Analytical Chemistry, vol. 5, pp. 293-315, Mar. 2012. [↩]
- K. Blagovic, L. Y. Kim, and J. Voldman, “Microfluidic perfusion for regulating diffusible signaling in stem cells,” PLoS ONE, vol. 6, p. e22892, Nov. 2011. [↩]
- L. M. Przybyla and J. Voldman, “Attenuation of extrinsic signaling reveals the importance of matrix remodeling on maintenance of embryonic stem cell self-renewal,” in Proc. Natl. Acad. Sci. U.S.A., 2012, vol. 109, pp. 835-840. [↩]