- Authors: J. Scholvin, A. N. Zorzos, C. G. Fonstad, E. S. Boyden
- Sponsorship: NIH, Paul Allen Foundation, MIT McGovern-Institute MINT Grant
Optogenetics is commonly used for precision modulation of the activity of specific neurons within neural circuits [] , but assessing the impact of optogenetic neuromodulation on the neural activity of local and global circuits remains difficult. Our collaborative team recently initiated a project (Scholvin et al., SFN 2011) to design and implement 3-D silicon-micromachined electrode arrays with customizable electrode locations, targetable to defined neural substrates distributed in a 3-D pattern throughout a neural network in the mammalian brain and compatible with simultaneous use of a diversity of existing light-delivery devices.
We have developed a series of innovations aimed at facilitating the scalability aspect of these probes, i.e., aspects of probe design that should enable them to scale to 1000 channels of neural recording or more. First, we have developed streamlined electrode fabrication methodologies that enable micromachined probes to be first fabricated using conventional silicon micromachining, then rapidly assembled into custom 3-D arrays, with semi-automated formation of the necessary electrical connections and mechanical constraints. Second, we have developed a set of surgical and insertion technologies towards the goal of enabling the insertion of electrode arrays with a high number of electrode shanks into the brain, while minimizing probe insertion damage. Finally, to facilitate scaling of the channel count beyond what is feasible with external amplifiers, we are exploring new approaches for integration of amplifier circuits directly on the probe arrays themselves, to remove bottlenecks associated with connecting of probes to the outside world.
-
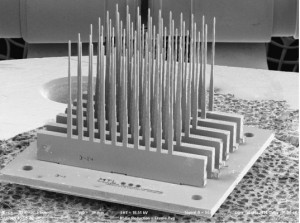
-
Figure 1: A scanning electron micrograph of six 10-probe linear arrays assembled into a 6 x 10 two-dimensional array of probes. The probes in this figure and in Figure 2 were fabricated for assembly studies and have no sense electrodes. Each needle is 3.5 mm long and 40 µm wide at the tip.
-

-
Figure 2: A close-up scanning electron micrograph of a portion of the array in Figure 1. The needles are thinned to 40 µm and are spaced 400 µm center-to-center in each linear array. The linear arrays are spaced at 600 µm. The above structure was fabricated using 300-µm thin wafers.
- Authors: A. Zorzos, J. Scholvin, C. G. Fonstad in collaboration with E. S. Boyden
- Sponsorship: McGovern Institute MINT Grant, NIH, Allen Foundation
Professor Ed Boyden uses light to precisely control neural activity. His lab has invented safe, effective ways to deliver light-gated membrane proteins to neurons and other excitable cells (e.g., muscle, immune cells, pancreatic cells, etc.) in an enduring fashion, thus making the cells permanently sensitive to being activated or silenced by millisecond-timescale pulses of blue and yellow light, respectively [] . This ability to modulate neural activity with a temporal precision that approaches that of the neural code itself holds great promise for human health, and his lab has developed animal models of epilepsy and Parkinson’s disease to explore the use of optical control to develop new therapies.
We have recently developed mass-fabricatable multiple light guide microstructures produced using standard microfabrication techniques to deliver light to activate and silence neural target regions along their length as desired [] . Each probe is a 100- to 150-micron-wide insertable micro-structure with many miniature lightguides running in parallel and delivering light to many points along the axis of insertion. Such a design maximizes the flexibility and power of optical neural control while minimizing tissue damage. We are currently developing 2-D arrays of such probes so multiple colors of light can be delivered to 3-dimensional patterns in the brain, at the resolution of tens to hundreds of microns, thus furthering the causal analysis of complex neural circuits and dynamics. Such devices will allow the substrates that causally contribute to neurological and psychiatric disorders to be systematically analyzed via causal neural control tools. Given recent efforts to test such reagents in nonhuman primates, these devices may also enable a new generation of optical neural control prosthetics, contributing directly to the alleviation of intractable brain disorders.
The initial light-guide structures have been fabricated from silicon oxynitride clad with silicon dioxide, and tests show excellent transmission of light with no visible loss in the taper and bend regions of the patterns [] . Significantly, the novel 90˚ bend invented to direct light laterally out the side of the narrow probe functions as designed [] . The optical sources for initial tests with the probe are independent laser modules coupled to one end of a fiber-optic ribbon cable (see Figure 2). The other end of the ribbon cable is butt-coupled to the inputs of the probe via a standard fiber-optic connector ferrule. This allows for increased modularity and control in initial probe testing.
We are now utilizing transgenic mice, which express optogenetic activators and silencers in cortical pyramidal neurons, to demonstrate optogenetic control of neural circuits in a fashion appropriate for in vivo circuit mapping or brain machine interface prototyping. Our goal is to explore the degree to which this technology can be used to functionally map neural network connectivity over large, multi-region circuits in the brain, and to subserve a new generation of neural control prosthetics.
-

-
Figure 1: Two free-standing light-guide probes. Each of these probes contains twelve waveguides, each 10 µm by 10 µm in cross-section, running from the input end (where they are spaced 125 µm center-to-center to match a fiber ribbon cable) to a unique 45˚ mirror reflector to direct the light laterally out of the probe.
-

-
Figure 2: A photograph of a light-guide probe (side view) mounted on a coupler ferrule attached to the output of a 12-fiber ribbon cable. Two of the guides on this side of the probe are excited, one with red light and the second with blue light. The emission from the side of the probe is visible in the picture.



