Stabilized CdSe-CoPi Composite Photoanode for Light-Assisted Water Oxidation by Transformation of Thin-Film CdSe/Cobalt Metal
- Category: Energy, Materials
- Tags: ronny costi, vladimir bulovic
Water oxidation is the thermodynamically demanding step of the water-splitting reaction. Efficient sunlight-driven water splitting into molecular oxygen and hydrogen is a challenging prerequisite for solar-fuels production [1] . For this prerequisite to be achieved, two major requirements are necessary: (1) efficient photon-to-charge conversion, with efficient utilization of the solar spectrum, and (2) a lowering of the energetic barriers for the four-proton, four-electron proton-coupled electron transfer (PCET) reaction of water splitting. The first requirement can be addressed by using semiconducting materials that absorb well into the visible range, such as cadmium chalcogenides. However, many of these materials including CdSe are unstable and oxidize rapidly under the aqueous conditions for water oxidation, rendering them unusable for water splitting purposes. The second requirement of lowering energetic barriers to water oxidation has been researched extensively in the last several decades to yield different forms of catalysts. One such catalyst is the cobalt-based water oxidation catalyst (CoPi) [2] . CoPi can be formed either via electrochemical deposition from Co2+ ions in aqueous solutions containing potassium phosphate (KPi) or by processing of supported thin-film (800 nm thick) cobalt metal anodes in KPi at pH 7 [3] .
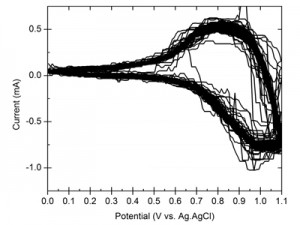
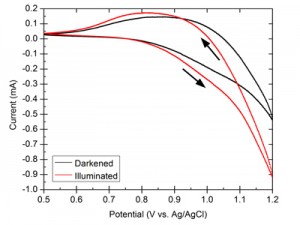
We demonstrate the dual benefit gained by using thin-film cobalt metal as the precursor in the preparation of CoPi on CdSe photoanodes. First, the cobalt layer protects the underlying semiconductor from oxidation and degradation in the aqueous solution. This process is followed by the advantageous incorporation of the CdSe layer into the CoPi layer during continued processing of the electrode. The resulting hybrid material forms a stable photoactive anode for light-assisted water oxidation. Figure 1 shows the high stability and reproducibility of currents through the hybrid material under demanding conditions. The benefits of the photoactive CdSe component are demonstrated in Figure 2, which presents the shift in catalytic onset under illumination.
- Figure 1: 1000 CV scans showing the stability of the CdSe/CoPi electrode in keeping the same currents over a span of 6 hours, demonstrating the high stability of the CdSe/CoPi electrode.
- Figure 2: CV showing the difference between a 1 cm2 CdSe/CoPi electrode in the dark (black) and under illumination (red), where the onset potential is lower and the currents are higher in the latter, due to the photo-assisted potential from the CdSe semiconductor.
- N. S. Lewis and D. G. Nocera, “Powering the planet: Chemical challenges in solar energy utilization,” Proc. Nat’l. Acad. Sci. USA, , vol. 103, pp. 15729-15735, Oct. 2006. [↩]
- M. W. Kanan and D. G. Nocera, “In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+,” Science, vol. 321, pp. 1072-1075, Aug. 2008. [↩]
- E. R. Young, D. G. Nocera and V. Bulović, “Direct formation of a water oxidation catalyst from thin-film cobalt,” Energy Environ. Sci., vol. 3, pp. 1726-1728, Nov. 2010. [↩]