Removal of Pathogen and Inflammatory Components from Blood using Cell Margination
- Category: MEMS & BioMEMS
- Tags: han wei hou, healthcare, jongyoon han
Sepsis is an adverse systemic inflammatory response caused by microbial infection in blood. In this work, we report a simple microfluidic approach for intrinsic, non-specific removal of both microbes and inflammatory cellular components (platelets and leukocytes) from whole blood, inspired by the in vivo phenomenon of leukocyte margination [1] . As blood flows through a narrow microchannel (20 × 20 µm), deformable red blood cells (RBCs) migrate axially to the channel center, resulting in margination of other cell types (bacteria, platelets and leukocytes) towards the channel sides (see Figure 1) [2] . With the use of a simple cascaded channel design, the blood samples undergo a 2-stage bacteria removal in a single pass through the device, thereby allowing higher bacterial removal efficiency. As an application for sepsis treatment, we demonstrated separation of Escherichia coli and Saccharomyces cerevisiae spiked into whole blood, achieving high removal efficiencies of ~80% and ~90%, respectively (Figure 2A). Inflammatory cellular components were also depleted by >80% in the filtered blood samples, which could help to modulate the host inflammatory response and potentially serve as a blood-cleansing method for sepsis treatment. The developed technique offers significant advantages including high throughput (~1mL/hr per channel) and label-free separation that allows non-specific removal of any blood-borne pathogens (bacteria and fungi). The continuous processing and collection mode potentially enables the return of filtered blood to the patient directly, similar to a simple and complete dialysis circuit setup. Due to design simplicity, further multiplexing is possible by increasing channel parallelization or device stacking to achieve higher throughput comparable to convectional blood dialysis systems used in clinical settings.
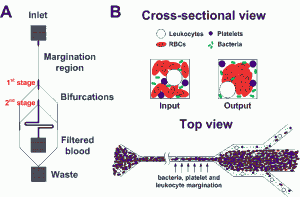
- Figure 1: Schematic illustrations of the (A) microfluidic design and (B) separation principle. As blood flows through a narrow microchannel (20 × 20 µm, W×H), deformable RBCs migrate axially to the channel center, resulting in margination of other cell types (bacteria, platelets and leukocytes) towards the channel walls and subsequently removal from the side outlets while the center outlet collects the bacteria-depleted blood.
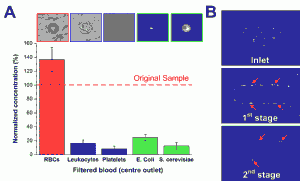
- Figure 2: (A) Normalized concentration of different cellular components at the filtered center outlet using human whole blood spiked with Escherichia coli and Saccharomyces cerevisiae. (B) Optical images illustrating yeast filtration (red arrows) at different stages.
- H. L. Goldsmith and S. Spain, “Margination of leukocytes in blood flow through small tubes,” Microvascular Research, vol. 27, pp. 204-222, 1984. [↩]
- H. W. Hou, H. Y. Gan, A. A. S. Bhagat, L. D. Li, C. T. Lim, and J. Han, “A microfluidics approach towards high-throughput pathogen removal from blood using margination,” Biomicrofluidics, vol. 6, pp. 024115-13, 2012. [↩]