Quantum Dot Light Emitting Diodes with an Electrophoretically Deposited Quantum Dot Layer
- Category: Electronic Devices, Materials, Nanotechnology
- Tags: katherine song, quantum dots, ronny costi, vladimir bulovic
Quantum dot light emitting diodes (QD-LEDs) are promising devices for the next generation of solid-state lighting and other optoelectronic applications. QD-LEDs have several potential advantages over current technologies due to the unique properties of quantum dots, such as a very narrow and easily tunable emission bandwidth, broad excitation spectrum, high brightness, and improved shelf life over organic dyes (used in organic LEDs) [1] [2] [3] . Quantum dot films for QD-LEDs are conventionally formed via spin-casting, which is a reliable but highly non-scalable process. To date, a few alternatives to spin-casting have been researched, but due to its simplicity, spin-casting remains the most common technique for forming dot films for QD-LEDs.
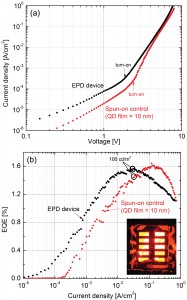
We investigated electrophoretic deposition (EPD) as an alternative method to spin-casting for the deposition of quantum dot films. Electrophoretic deposition is an experimentally simple, well-established technique that has been used to deposit a variety of materials [4] . In addition to offering the potential for parallel processing and for less material waste during processing, EPD could potentially create more ordered films than spin-casting. We fabricated QD-LEDs (Figure 1) with an electrophoretically deposited CdSe/ZnS core-shell QD film. EPD is performed by submerging 2 ZnO-on-ITO electrodes into a solution of QDs in a sonication bath and applying a DC field of ~25 V/cm for 5 minutes between the electrodes. Completed QD-LEDs fabricated with an electrophoretically deposited dot layer exhibited sub-bandgap turn-on voltages of ~1.8 V and peak external quantum efficiencies (EQE) of ~1.6%, a number comparable to that of QD-LEDs fabricated with a conventional spun-on dot layer (Figure 2). These findings demonstrate that EPD is a viable alternative to spin-casting for the large-area, high-throughput fabrication of QD-LEDs with respectable performance.
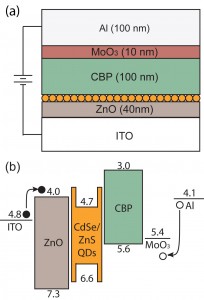
- Figure 1: (a) Cross-sectional schematic and (b) energy band diagram of a completed QD-LED.
- Figure 2: (a) Current density vs. voltage and (b) external quantum efficiency vs. current density for a QD-LED with an electrophoretically deposited QD layer (inset: 10 devices operating simultaneously at ~100 cd/m2 in the dark).
- C. B. Murray, D. J. Norris, and M. G. Bawendi, “Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites,” J. Am. Chem. Soc., vol. 115, no. 19, pp. 8706-8715, 1993. [↩]
- B. O. Dabbousi, J. Rodriguez Viejo, F. V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K. F. Jensen, and M. G. Bawendi, “(CdSe)ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites,” J. Phys. Chem. B, vol. 101, no. 46, pp. 9463-9475, 1997. [↩]
- P. O. Anikeeva, J. E. Halpert, M. G. Bawendi, and V. Bulović, “Electroluminescence from a mixed red-green-blue colloidal quantum dot monolayer,” Nano Lett., vol. 7, no. 8, pp. 2196-2200, 2007. [↩]
- O. O. Van der Biest and L. J. Vandeperre, “Electrophoretic deposition of materials,” Annu. Rev. Mater. Sci., vol. 29, pp. 327-352, 1999. [↩]