Optimization of Single- and Multi-wall Carbon Nanotube Growth on Nanoscale Zirconia and Other Oxides as Non-metallic Catalysts
- Category: Nanotechnology
- Tags: akira kudo, brian wardle
This work focuses on the development of non-metallic substances that can catalyze carbon nanotube (CNT) growth while remaining in a non-metallic state and the optimal conditions to obtain the best CNT growth yield utilizing such non-metallic substances. In our group’s previous work [1] , we successfully demonstrated that a non-metallic catalyst, nano-particulated zirconia, can catalyze CNT growth by thermal chemical vapor deposition (CVD) while remaining in an oxidized state. This novel class of catalyst for CNT growth will impact the potential to grow CNTs on historically challenging substrates, further expanding applications of CNTs. Since this discovery, extensive studies have clarified the mechanisms through which these non-metallic nanoparticles work as catalysts, as well as the optimal conditions for them to obtain high yield growth.
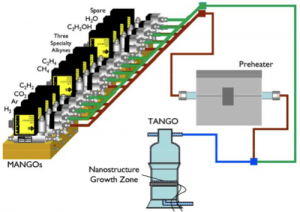
In order to smoothly perform our parametric approach, we have built a new CVD reactor and mass-flow controller array system (Figure 1). MANGO-TANGO, the acronym for mass-flow array for nanotube growth optimization and table-top apparatus for nanotube growth optimization, has versatile capabilities such as handling twelve gas species at once, precise measurement of reaction spot temperature, and selective thermal treatment of gas species. Utilizing the new mass-flow controller array system (MANGO), we have confirmed that carbon xerogel–a type of nanoporous and amorphous carbon that is easy to fabricate–is as active a substrate as carbon aerogel for zirconia nanoparticles.
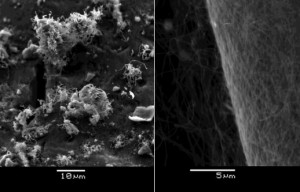
It has been understood that the precursor for non-metallic catalysts affects the morphology of resulting CNT growth. For example, both ready-made zirconia nanoparticles and zirconium oxychloride solutions work as good sources of catalysts, but the growth morphologies from them are substantially different (Figure 2). We believe that our research employing more characterization techniques such as TEM, XPS, and Raman spectroscopy will provide important insights into the science behind CNT growth and the engineering applications of CNT.
- Figure 1: Schematic of MANGO-TANGO equipment [2] .
- Figure 2: CNTs grown on carbon aerogels with ready-made zirconia nanoparticles (left) and with zirconium oxychloride (right). Ready-made zirconium nanoparticles grew thick and short CNTs, while zirconium oxychloride grew thin and long CNTs [2] .
- S. A. Steiner III, T. F. Baumann, B. C. Bayer, R. Blume, M. A. Worsley, W. J. MoberlyChan, E. L. Shaw, R. Schloegl, A. John Hart, S. Hofmann, and B. L. Wardle, “Nanoscale zirconia as a nonmetallic catalyst for graphitization of carbon and growth of single- and multiwall carbon nanotubes,” Journal of the American Chemical Society, vol. 131, no. 34, pp. 12144-12154, 2009. [↩]
- S. A. Steiner III, Ph.D. dissertation, Massachusetts Institute of Technology, Cambridge, 2011. [↩] [↩]