Ultrahigh Resolution Fluorescence Microscope Utilizing a J-aggregate Critically Coupled Resonator
- Category: Nanotechnology, Optics & Photonics
- Tags: Vladimir Bulovic, William Tisdale
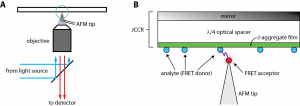
Figure 1: Schematic of the scanning near-field optical microscope utilizing FRET and a JCCR. (A) The sample is illuminated from the front of the JCCR through an objective lens. Fluorescence is collected through the same objective and a transparent non-interfering AFM probe tip is used. (B) Magnified cartoon of the tip-sample interaction in (A). The JCCR is frequency-matched to the absorption band of the analyte to be detected (a fluorescing small molecule, nanoparticles, or protein of interest). Light is absorbed in the J-aggregate thin film, and then its energy is transferred to the analyte. A fluorescence moiety is attached to the AFM probe tip. When the tip comes within the Förster radius of the analyte (typically 2-5 nm), fluorescence from the probe is detected in the far field.
The non-destructive chemical mapping of surfaces with single-molecule sensitivity and sub-5-nm lateral resolution is an extraordinary challenge. Optical techniques such as absorption, fluorescence, or Raman spectroscopy are powerful tools for chemical detection because of the unique optical signature that each molecule possesses. However, the maximum resolution of a classical (far-field) light microscope is limited by the fundamental law of diffraction. Typically, the resolving power of visible light cannot be better than ~200-300 nm in the lateral dimensions (x and y) and ~500-800 nm axially (z) [1] . Unfortunately, almost all biomolecules and chemical agents of interest are smaller than this resolution limit. To overcome these classical limitations, we are developing an ultrabright near-field fluorescence microscope capable of single-molecule detection sensitivity, chemical specificity, and sub-5-nm lateral resolution. Ultrahigh spatial resolution is achieved by utilizing a strongly distance-dependent near-field optical interaction called Förster resonance energy transfer (FRET). FRET is the near-field transfer of energy from a photoexcited fluorophore (the “donor”) to another nearby fluorophore (the “acceptor”). By attaching the acceptor species to a scanning probe tip, donor fluorophores can be spatially located on the sample surface within the Förster radius of the interaction (typically 2-5 nm, see Figure 1). Because single molecule fluorescence signals are weak, we employ an optical structure referred to as a J-aggregate critically coupled resonator (JCCR) to enhance the brightness of the donor [2] . The JCCR, which consists of a mirror, a λ/4 optical spacer, and a thin film (<15 nm thick) of J-aggregating dye molecules (see Figure 1), serves as a very general platform for efficient light capture and redistribution. Greater than 95% of incident light is absorbed within the thin J-aggregate film and then transferred to the donor species residing on the JCCR surface for subsequent FRET to the scanning probe tip.