Single-cell Trapping and DNA Damage Analysis Using Microwell Arrays
- Category: MEMS & BioMEMS
- Tags: Bevin Engelward, Jing Ge
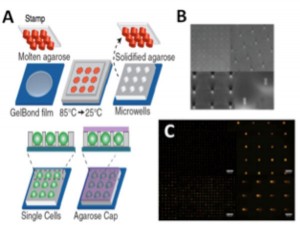
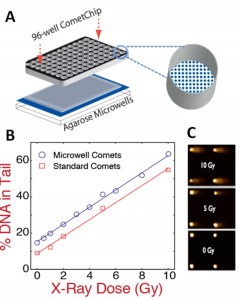
DNA damage has been found to play critical roles in cancer, aging, and heritable disease. There is rising interest in studying DNA damage and repair kinetics in cells, but the lack of a robust, inexpensive, and high-throughput device for quantitative DNA damage analysis makes such investigations far from routine. The single cell gel electrophoresis or “comet” assay is one of the best-established methods for detection of DNA lesions and strand breaks. Based on the principle that relaxed loops and fragments of damaged DNA migrate farther under the influence of an electrical field in agarose gel than undamaged DNA, the level of DNA damage can be assessed by measuring the relative amount of DNA migration. Although extremely versatile and inexpensive, the comet assay is restrained from wider acceptance due to its low throughput, poor reproducibility and laborious and potentially biased analysis methods. Through incorporation of microfabrication techniques, we have developed a microarray platform to perform high throughput single cell electrophoresis with improved consistency. Different from randomly dispersed cells in the traditional comet assay, cells on our platform are patterned into spatially registered microscopic wells. These microwells are formed by direct stamping of hydrated gels with molds that contain patterned microposts fabricated through Su-8 photolithography (Figure 1). Cells are arrayed through passive settling into the microwells and can then undergo treatment and analysis. We have also developed software that utilizes the unique patterning feature to automatically analyze images with high accuracy and reproducibility (Figure 1). By sandwiching our patterned agarose gel between a bottomless 96-well plate and glass plate, we have transformed our assay into a multiwell version, referred to as “the CometChip.” This 96-well format enables simultaneous investigation of different chemical conditions among different cells samples, as well as analysis of repair kinetics. Importantly, the CometChip is compatible with standard automated liquid handling and imaging. The efficacy and increased throughput of the CometChip for DNA damage analysis is demonstrated by comparing an irradiation dose response to the traditional comet assay (Figure 2). All doses and replicates were assayed on a single CometChip in significantly shorter time and with less labor. Our research has demonstrated a significant technological advance to traditional methodology and opened countless possibilities in epidemiology and drug development applications.
- Figure 1: Single cell capturing and gel electrophoresis in microarrays. (A) Microwell fabrication. A microfabricated stamp is placed onto molten agarose. Agarose is cooled to set, and the stamp is removed. Cells are loaded into wells by gravitational settling, and an agarose overlay covers the cells. (B) Scanning electron micrograph of SU-8 posts patterned onto a silicon substrate. (C) Arrayed microwell comets. Top: undamaged; Bottom: damaged.
- Figure 2: Multiwell comet array. (A) Assembly of 96-well comet array. Agarose gel with microwells is sandwiched between a glass substrate and a bottomless 96-well plate and sealed with mechanical force. (B) Comparison of irradiation dose response between traditional comet slides and microwell comets. Each data point is the median of 50 individual comets. (C) Microwell comets with varying doses of irradiation damage.