Nanoscale Zirconia and Other Oxides as Non-metallic Catalysts for Graphitization of Carbon and Growth of Single- and Multi-wall Carbon Nanotubes
- Category: Nanotechnology
- Tags: Brian Wardle, Stephen Alan Steiner III
This work focuses on development of non-metallic substances that can catalyze carbon nanotube (CNT) growth while remaining in a non-metallic state.
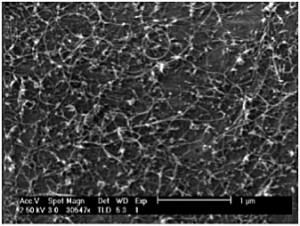
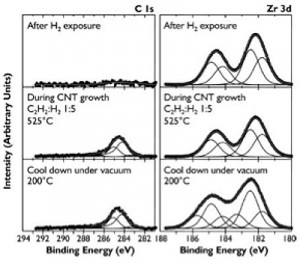
We recently reported that nanoparticulate zirconia (ZrO2) catalyzes both growth of single-walled CNTs and multi-walled CNTs by thermal chemical vapor deposition (CVD) and graphitization of solid amorphous carbon [1] . We observe (Figure 1) that silica-, silicon nitride-, and alumina-supported zirconia on silicon nucleates single- and multiwall CNTs upon exposure to hydrocarbons at moderate temperatures (750°C). Using in situ, high-pressure, time-resolved X-ray photoelectron spectroscopy (XPS) of these substrates during CNT nucleation and growth (Figure 2), we show that the zirconia catalyst neither reduces to a metal nor forms a carbide. Point-localized energy-dispersive X-ray spectroscopy using scanning transmission electron microscopy confirms that catalyst nanoparticles attached to CNTs are zirconia. We also observe that carbon aerogels containing zirconia nanoparticles prepared through pyrolysis of a Zr(IV)-containing resorcinol-formaldehyde polymer aerogel precursor at 800°C contain fullerenic cage structures absent in undoped carbon aerogels. Zirconia nanoparticles embedded in these carbon aerogels are further observed to act as nucleation sites for multi-walled CNT growth upon exposure to hydrocarbons at CVD growth temperatures.
Our study clearly demonstrates, for the first time, that a non-metallic catalyst can catalyze CNT growth by thermal CVD while remaining in an oxidized state. Currently, the yield of CNTs obtained from zirconia is significantly lower than from traditional transition metal catalysts such as Fe. Studies to identify how to optimize yield from oxide catalysts not reducible under CVD growth conditions, the role of defects in these catalysts, and the sensitivity of these catalysts to different gas-phase species are underway. We believe that characterization of the mechanisms underlying CNT growth from oxides will aid in the engineering of optimized non-metallic catalysts for CNT growth on historically challenging substrates such as carbon fiber and may offer a promising route towards control of CNT and other nanostructure characteristics.
- Figure 1: SEM images of CNTs grown from zirconia on a Si wafer with 10-nm alumina support, analyzed by XPS in situ during CVD.
- Figure 2: Progressive XPS snapshots of the C 1s and Zr 3d regions during growth of CNTs from zirconia on Si substrate. An appreciable carbon signal only appears upon introduction of both acetylene and hydrogen. No metallic zirconium or zirconium carbide is observed during growth.
- S. A. Steiner III, T. F. Baumann, B. C. Bayer, R. Blume, M. A. Worsley, W. J. MoberlyChan, E. L. Shaw, R. Schloegl, A. John Hart, S. Hofmann, and B. L. Wardle, “Nanoscale zirconia as a nonmetallic catalyst for graphitization of carbon and growth of single- and multiwall carbon nanotubes,” Journal of the American Chemical Society, 2009 vol. 131, no. 34, pp. 12144-12154. [↩]