Nano Line Fracture Sensor for Explosive Detection
- Category: Nanotechnology
- Tags: George Barbastathis, Hyungryul Choi, Karen Gleason
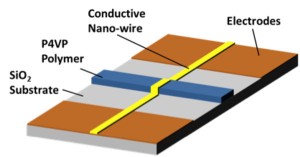
Selective detection of explosive compounds is critical for national defense and homeland security. Nitroaromatic compounds pose a particular threat; 2,4,6-trinitrotoluene (TNT), for example, is an inexpensive and readily available component of fifteen of the most widely used blends [1] . Existing methods to detect explosives include biosensors [2] , electrochemical sensors [3] and fiber optic [4] sensors. Devices utilizing chromatography [5] and Raman spectroscopy [6] are used for the same purpose. However, sensors using the aforementioned techniques require complicated sensing and readout components; moreover, they are comparatively large in size and consume significant amounts of power during operation. In this work we describe the fabrication and demonstration of a chemical sensor capable of detecting nitroaromatic explosives in air. The aim of this work is the development of a simple sensor that has the unique features of micro-scale dimensions, simple and inexpensive fabrication, and low power consumption. It consists of a nano-patterned conductive metal line placed on top of a patterned responsive polymer, poly(4-vinylpyridine) (P4VP), as shown in Figure 1. Due to polymer-solvent interactions, P4VP swells when it encounters the target analyte, producing a large stress. Detection takes place by monitoring the change in device resistance as the metal nano line deforms or fractures when P4VP swells and transfers mechanical stress.
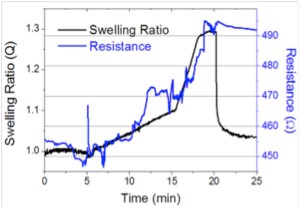
Fabricated devices were tested for their response to nitroaromatic exposure using a previously described system((W. E Tenhaeff, L. D. McIntosh, and K. K. Gleason, “Synthesis of poly(4-vinylpyridine) thin films by initiated chemical vapor deposition (iCVD) for selective nanotrench-based sensing of nitroaromatics,” Advanced Functional Materials, vol. 20, no. 7, pp. 1144-1151, 2010.)) [7] . Test devices were located on a cooled stage within a flow cell; swelling responses of P4VP films were measured via in situ interferometry. Figure 2 illustrates the change in device resistance for a 200-nm-thick, 5-μm-wide P4VP line intersected by a 100-nm-thick, 300-nm-wide Au line sensor upon exposure to 500 ppm of nitrobenzene. The concentration was increased to 650 ppm at t=15mins. The change in resistance corresponds well to the calculated change in exposure concentration. A permanent increase (8.5%) in resistance is clearly observed as the result of permanent deformation and micro-cracks; this change is large enough to be easily detected.
- Figure 1: A schematic of the nano fracture chemical sensor.
- Figure 2: Change in device resistance of a 100-nm-thick, 300-nm-wide Au line sensor upon exposure to 500 ppm of nitrobenzene. The concentration was increased to 650 ppm at t=15 mins.
- S. J. Toal, and W. C. Trogler, “Polymer sensors for nitroaromatic explosives detection,” Journal of Materials Chemistry, vol. 16, no. 28, pp. 2871-2883, Apr. 2006. [↩]
- R. M. Wadkins, J. P. Golden, L. M. Pritsiolas, F., and S. Ligler, “Detection of multiple toxic agents using a planar array immunosensor,” Biosensors and Bioelectronics, vol. 13, no. 3, p. 407, 1998. [↩]
- K. Masunaga, K. Hayama, T. Onodera, K. Hayashi, N. Miura, K. Matsumoto, and K. Toko, “Detection of aromatic nitro compounds with electrode polarization controlling sensor,” Sens. Actuators B, vol. 108, nos. 1-2, p. 427, 2005. [↩]
- R. A. Ogert, L. C. Shriver-Lake, and F. S. Ligler, “Toxin detection using a fiber-optic-based biosensor,” in Proc. SPIE, 1993, vol. 1885, Mar. 1993, p. 11-17. [↩]
- A. Hilmi, J. H. T. Luong, and A. L. Nguyen, “Determination of explosives in soil and ground water by liquid chromatography–amperometric detection,” Journal of Chromatogr. A, vol. 844, nos. 1-2, p. 97, 1999. [↩]
- I. R. Lewis, N. W. Daniel Jr., N. C. Chaffin, P. R. Griffiths, and M. W. Tungol, “Raman spectroscopic studies of explosive materials: towards a fieldable explosives detector,” Spectrochimica Acta Part A, vol. 5, no. 12, p. 1985, 1995. [↩]
- W. J. Arora, W. E. Tenhaeff, K. K. Gleason, and G. Barbastathis., “Integration of reactive polymeric nanofilms into a low-power electromechanical switch for selective chemical sensing,” Journal of Microelectromechical Systems, vol. 18, no. 1, pp. 97-102, 2009. [↩]