Microfluidic Platforms for Studying the Role of the Biophysical and Cellular Microenvironment in Tumor Invasion
- Category: MEMS & BioMEMS
Tumor invasion has received considerable attention as a critical step in cancer metastasis, and is a promising target for developing new cancer drugs. Current understanding of the role of the biophysical and cellular microenvironment in tumor invasion is limited, because of the lack of appropriate in vitro and in vivo models. We have adapted our previous microfluidic platforms [1] for studying the role of the endothelium on tumor intravasation (entry into the vascular system) and the effects of interstitial flow on tumor cell migration, along with the development of new hard plastic devices for commercial transition.
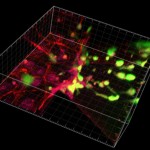
Recent results from the tumor-endothelial interaction assay demonstrated the capability to form a confluent endothelial monolayer on collagen type I matrices, in the presence of invading tumor cells in 3D (Figure 1). Stimulating the layer with inflammatory cytokines, we demonstrated an increase in diffusive permeability to fluorescent dextrans, in agreement with a measured increase in the number of intravasation events. These results demonstrate the utility of this assay for studying the role of the endothelial barrier function in tumor cell intravasation.
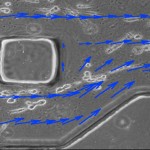
We also developed a microfluidic system for investigating the role of interstitial flow in tumor cell migration (Figure 2). Tumor cells exposed to interstitial flow preferentially migrated along streamlines, and the relative percentage of cells migrating upstream and downstream was found to be a function of chemokine receptor activity and cell density. Interstitial flow stimulated downstream tumor cell migration through CCR7-mediated autocrine signaling. However, flow also stimulated upstream cell migration through a competing, mechanically mediated pathway, as evidenced by significant increases in FAK activation in devices with flow. Relative strengths of the autocrine and mechanical stimuli determine whether cells migrate upstream or downstream.
We applied known commercially-viable manufacturing methods to a cyclic olefin copolymer (COC) to fabricate a microfluidic device with controlled surface properties and improved potential for high-volume applications. Culture of cells in the new COC device indicated no adverse effects. Therefore, this transition of platform demonstrates a capability of using microfludic devices for 3D cell culture across the range from the scientific research to applications with broad clinical impact.
- Figure 1: Confocal 3D rendering of tumor cells (green) migrating into the vicinity of an endothelial monolayer (red).
- Figure 2: Microfluidic system for investigating interstitial flow and effects on cell migration. Cells were suspended in 3D collagen gel, aligned with flow streamlines and migrated along streamlines. Blue arrows indicate local stream vectors. Post is 250 μm.
- S. Chung, R. Sudo, P. J. Mack, C. R. Wan, V. Vickerman, and R. D. Kamm. “Cell migration into scaffolds under co-culture conditions in a microfluidic platform.” Lab Chip, vol. 9, pp. 269-275, 2009. [↩]