Microfluidic Perfusion for Modulating Stem Cell Diffusible Signaling
- Category: MEMS & BioMEMS
- Tags: Joel Voldman, Katarina Blagovic, Laralynne Przybyla
Stem cell phenotype and function are influenced by microenvironmental cues that include cell-cell, cell-extracellular matrix (ECM), and cell-media interactions, as well as mechanical forces. Our research focuses on developing microscale systems for controlling the cellular microenvironment of mouse embryonic stem cells (mESCs), in particular mechanical forces (i.e., shear stress) and cell-media interactions (i.e., diffusible signaling).
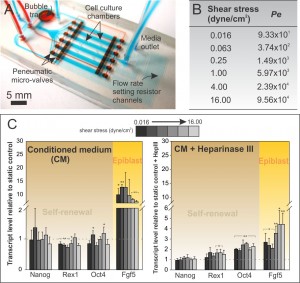
Many emerging technologies used for ESC expansion or differentiation require perfusion culture, an example being pluripotent stem cell expansion in bioreactors for clinical applications [1] . We employ a multiplex microfluidic perfusion array to study the effects of shear stress on mESCs across a wide range of flow rates in a graded, quantitative manner. Using this device, we are able to show that perfusion elicits phenotypic changes and that the specific shear-responsive phenotype is due to mechanosensing by heparan sulfate proteoglycans (HSPGs, Figure 1A-C). This is the first study describing the ESC machinery capable of responding to shear stress, thus providing a foundation for further shear mechanotransduction studies [2] .
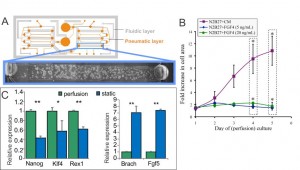
Cells are constantly secreting and responding to soluble signals, the removal of which can be mediated by modulating flow properties at the microscale. To assess the contribution of cell-secreted factors to mESC differentiation and self-renewal, we utilized a two-layer microfluidic perfusion device allowing for parallel comparison of different cell culture conditions (Figure 2A) [3] . Our results demonstrate that mESCs do not grow in differentiation conditions with minimal autocrine signaling, even with supplementation by Fgf4, a signal that has been shown to be a crucial factor in differentiation toward a neuronal stem cell fate (Figure 2B). Conversely, under self-renewal conditions, mESCs proliferate but lose self-renewal markers and upregulate differentiation markers (Figure 2C). These results, together with signaling and downstream differentiation assays, indicate that a differentiation towards an epiblast-like early differentiation state under conditions that had previously been shown as sufficient for self-renewal. Together, these results indicate the importance of cell-secreted signals for mESC growth and self-renewal.
- Figure 1: (A) Microfluidic perfusion array for probing shear stress effects on mESCs. (B) Range of shear stress used in this study and corresponding Peclet number (Pe). (C) Gene profile for a range of applied shear values under conditions of saturated soluble environment (CM) in the presence or absence of HSPG disruption.
- Figure 2: (A) Schematic of microfluidic perfusion device and mESCs growing in a single chamber in self-renewing medium. (B) Growth analysis of perfusion cultures in differentiation medium (N2B27) supplemented with Fgf4, and soluble factors saturated medium (N2B27+CM). (C) Expression levels of self-renewal (left) and differentiation (right) markers of mESCs growing in self-renewal medium in the device shown in (A) versus mESCs grown in a standard culture dish.
- D. Steiner, H. Khaner, M. Cohen, S. Even-Ram, Y. Gil, P. Itsykson, T. Turetsky, M. Idelson, E. Aizenman, R. Ram, Y. Berman-Zaken, and B. Reubinoff, “Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension,” Nature Biotechnology, vol. 28, pp. 361-364, Mar. 2010. [↩]
- Y.-C. Toh and J. Voldman, “Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction,” The FASEB Journal, vol. 25, pp. 1208-1217, 2011. [↩]
- K. Blagović, L.Y. Kim, A. M. Skelley, and J. Voldman, “Microfluidic control of stem cell diffusible signaling,” in Proc. Twelfth International Conference on Miniaturized Systems for Chemistry and Life Sciences, San Diego, USA, pp. 677-679, Oct. 2008. [↩]