Integration of Cobalt-based Catalyst with Silicon Photoanodes to Achieve Photo-assisted Water Oxidation
- Category: Energy, Materials
- Tags: Elizabeth Young, Vladimir Bulovic
Transformation of solar energy into chemical fuels is an attractive energy conversion transformation to address the intermittency of power generation in photovoltaics, which has been a long-standing challenge for development of solar energy [1] . Stored solar fuels may then be used when sunlight does not reach the solar panels, enabling continuous, solar-generated energy availability. The fuel cycle addressed in this work is the splitting of water to molecular hydrogen and oxygen, which can be used as fuels or as high-energy precursors in fuel synthesis.
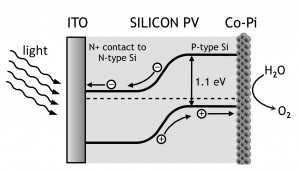
We utilize a cobalt-containing water oxidation catalyst (Co-Pi) that assists the water to oxygen reaction [2] . Co-Pi, can be formed either via electrodeposition from Co2+ ions in aqueous solutions containing potassium phosphate (KPi) or by processing supported thin-film (800 nm thick) cobalt metal anodes in KPi at pH 7. Here, we use doped silicon wafers, Figure 1, as substrates for either electrodeposition of Co-Pi or processing of cobalt metal thin films into Co-Pi catalyst. Photoanode structures of ITO/Si/Co/Co-Pi, ITO/Si/ITO/Co-Pi and ITO/Si/ITO are prepared and tested for photo-assisted water oxidation activity.
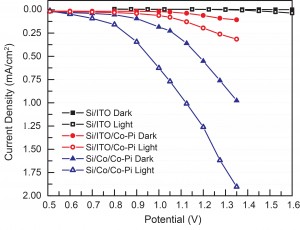
Differences in water oxidation performance of each photoanode are particularly clear in Figure 2, which shows a comparison of steady-state current versus applied potential under dark and light conditions. The ITO/Si/ITO electrode passes very little current (10 μA/cm2) compared to the Co-Pi loaded electrodes and shows negligible change between light and dark conditions. The ITO/Si/ITO/Co-Pi electrode exhibits increasing dark current densities as voltage is applied and demonstrates additional increase under illumination (current offset between dark and light conditions reaches 200 μA/cm2 at 1.35 V). The ITO/Si/Co/Co-Pi electrode achieves current densities higher than the ITO/Si/ITO/Co-Pi electrode in both dark and light conditions. The dark/light current offset over the catalytic regime of the electrode (between 0.85 V to 1.35 V) increases from 100 μA/cm2 (at 0.85 V) to 1 mA/cm2 (at 1.35 V) and even higher. These results demonstrate the importance of material selection and device engineering for harnessing the solar spectrum towards catalytic chemical fuel production. The robust and abundant silicon electrode and direct processing of the cobalt metal produces an improved light-assisted water oxidation device.
- Figure 1: Schematic of the silicon P-N junction photovoltaic device used as a photoanode coated with the thin film of Co-Pi catalyst on the P-side and the ITO electrode on the N-side.
- Figure 2: The graph plots the steady-state current density at each applied potential. The ITO/Si/ITO electrode shows minimal activity compared to the Co-Pi loaded electrodes. Under illumination the ITO/Si/Co/Co-Pi electrode has significantly higher current densities than ITO/Si/ITO/Co-Pi, emphasizing that the judicious choice of materials and their sequencing on the photoanodic substrate are of critical importance.
- T. R. Cook, D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets and D. G. Nocera, “Solar energy supply and storage for the legacy and nonlegacy worlds,” Chem. Rev. vol. 110, pp. 6474-6502, 2010. [↩]
- M. W. Kanan and D. G. Nocera, “In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+,” Science vol. 321, pp. 1072-1075, 2008. [↩]