Formation of Organic Molecular Nano-crystals on Engineered Surfaces
- Category: Nanotechnology
- Tags: Allan Myerson, Xiaochuan Yang
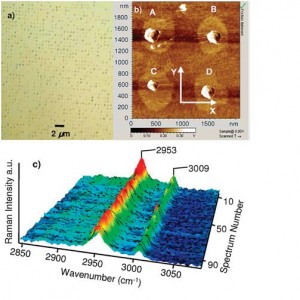
Figure 1: (a) Optical microscope image (×150) of 1600 µm2 and (b) AFM image of 4 µm2 of the 500-nm patterned SAMs on the silicon substrate. The crystals dimensions are: (A) 250 × 220 × 74 nm3, (B) 230 × 205 × 70 nm3, (C) 180 × 160 × 57 nm3, and (D) 220 × 190 × 65 nm3. (c) Raman spectra of 100 individual glycine crystals found on the patterned SAMs. All crystals were determined to be β-form [1] .
The formation of organic molecular nano-crystals is a topic of great interest in the pharmaceutical industry because of the potential increase in solubility of organic crystals below 1 micron and their potential use in nano-suspensions for direct injection. Direct production of nano-crystals through crystallization while controlling the crystal form (polymorph) is a difficult problem and a current active area of research. Investigators have explored methods based on the use of confinement (such as nano-wells), surface templating (such as polymers and self-assembled-monolayers (SAMs), and microfluidics [1] [2] [3] [4] [5] [6] [7] [8] [9] . Our group has developed a method to “create a confined volume” for crystallization on templated surfaces (Figure 1), which results in both a confined volume and a templating effect [10] [11] .
In our research, we fabricate both hydrophilic and hydrophobic thiol SAMs on gold surfaces. With the use of photolithography or electron beam lithography, desired patterns of hydrophilic and hydrophobic SAMs can be generated, allowing the formation of small droplets of solution on the hydrophilic surfaces. By controlling the solution conditions through cooling evaporation or vapor diffusion of an anti-solvent, we can control the supersaturation profile and thus induce nucleation and growth of crystalline solids.
Our current work employs patterns of SAMs allowing the formation of droplets as small as 100 nm in diameter. Using these droplets, we are probing issues related to nucleation and polymorphism in a number of different systems to better understand the fundamentals of nucleation and polymorphism.
- C. Price, A. Grzesiak, and A. Matzger, “Crystalline polymorph selection and discovery with polymer heteronuclei,” J. Am. Chem. Soc., vol. 127, pp. 5512-5517, 2005. [↩] [↩]
- P. Carter and M. Ward, “Directing polymorph selectivity during nucleation of anthranilic acid on molecular substrates,” J. Am. Chem. Soc., vol. 116, pp. 769-770, 1994. [↩]
- R. Hiremath, J. Basile, S. Varney, and J. Swift, “Controlling molecular crystal polymorphism with self-assembled monolayer templates,” J. Am. Chem. Soc., vol. 127, pp. 18321-18327, 2005. [↩]
- V. Genota, S. Desportesb, C. Croushorea, J. Lefevrea, R. Pansua, J. Delairea, P. von Rohr , “Synthesis of organic nanoparticles in a 3D flow focusing microreactor,” Chemical Engineering Journal, vol. 161, pp. 234-239, 2010. [↩]
- C. Hansen, S. Classen, J. Berger, and S. Quake, “A microfluidic device for kinetic optimization of protein crystallization and in situ structure determination,” Journal of the American Chemical Society, vol. 128, pp. 3142-3143, 2006. [↩]
- C. Hansen, E. Skordalakes, J. Berger, and S. Quake, “A robust and scalable microfluidic metering method that allows protein crystal Growth by Free Interface Diffusion,” Proc. National Academy of Sciences of the United States of America, vol. 99, pp. 16531-16536, 2002. [↩]
- C. Jackson and G. McKenna, “Vitrification and crystallization of organic ;iquids confined to nanoscale pores,” Chem. Mater., vol. 8, pp. 2128-2137, 1996. [↩]
- L. Wang, M. Lee, J. Barton, L. Hughes, and T. Odom, “Shape-control of protein crystals in patterned microwells,” J. Am. Chem. Soc., vol. 130, pp. 2142-2143, 2008. [↩]
- E. You, R. Ahn, M. Lee, M. Raja, T. O’Halloran, and T. Odom, “Size control of arsenic trioxide nanocrystals grown in manowells,” J. Am. Chem. Soc., vol. 131, pp. 10863-10865, 2009. [↩]
- K. Kim, I. Lee, A. Centrone, T. Hatton, and A. Myerson, “Formation of nanosized organic molecular crystals on engineered surfaces,” J. Am. Chem. Soc., vol. 131, pp. 18212-18213, 2009. [↩]
- K. Kim, A. Centrone, A. Hatton, and A. Myerson, “Polymorphism control of nanosized glycine crystals on engineered surfaces,” Cryst. Eng. Comm., vol. 13, pp. 1127-1131, 2011. [↩]