Dynamic Cell Deformability Study in Microfluidic Devices
- Category: MEMS & BioMEMS
- Tags: Jongyoon Han, Sha Huang
The mechanical properties of tissues and cells have important implications on their differentiated state, functions and responses to injury. Altered cell deformability is both a cause of and biomarker for potentially severe diseases, such as cancer, sickle cell anemia and malaria. In the past, several techniques have been developed to measure single-cell deformability including micropipette aspiration, atomic force microscopy, and optical tweezers. However, many of these measurements assess only static cell deformations which often fail to reflect in vivo situation when cells are in microcirculation. Additionally, the low throughput of the techniques limits sampling size per experiment, which may potentially lead to misrepresentation of population-wide trait. Therefore, we aim to develop a microfluidic device which measures cell dynamic deformability with high sensitivity and high throughput.
In this project, the relation between cell dynamic deformability and disease state is aimed to be established for several representative cell lines including human erythrocytes, breast cancer cells, and mesenchymal stem cells. The impact of microenvironmental controls such as temperature fluctuation and drug treatment on the deformability of malaria infected cells is also investigated.
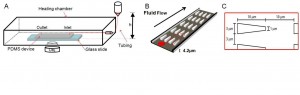
- Figure 1: A) Experimental schematics. A heating chamber (Olympus) was mounted to the inverted microscope stage. B) The PDMS-glass bonded device consists of the inlet and outlet reservoirs and main channels with triangular pillar arrays. C) Design parameter for red blood cell experiment. Along the flow direction, the inter-pillar spacing was 10 µm. This spacing would allow deformed cells to recover and ready for subsequent deformations. Perpendicular to the flow direction, the pore size varied from 2.5 to 4 µm for different channels.
- Figure 2: A) Healthy RBC velocity vs. temperature plot. Approximately 1000 cells were tracked. B) Ring stage infected RBCs passing through 3µm channels. The temperature effect in cell stiffening is illustrated. C) Both infected RBCs and co-cultured uninfected RBCs are exposed to artesunate drug at 0.01, 0.05 and 0.10 µg/ml concentration for 2, 4 and 6 hours. The drug effect is assessed at 37.0˚C. Notations: black circles represent uninfected RBCs, red circle represe˚nt infected cells and blue circles represent healthy cells