Continuous Signal Enhancement for Sensitive Aptamer Mobility Shift Assay Using Electrokinetic Concentration
- Category: Medical Electronics, MEMS & BioMEMS
- Tags: Jongyoon Han, Lih Feng Cheow
Aptamers are emerging as popular alternatives to antibodies as affinity probes in immunoassays. From a point-of-care diagnostics standpoint, aptamers have an advantage over antibodies since they are stable over a wide range of conditions and can be chemically synthesized at low cost. Affinity probe capillary electrophoresis (CE) [1] [2] is a promising platform with which to perform aptamer-based biomarker detection as it features fast homogeneous reaction kinetics and requires only one affinity probe species, although sensitivity is still limited due to band dispersion, complex dissociation and lack of amplification reaction.
We have previously demonstrated microfabricated nanofluidic preconcentration devices that can continuously accumulate a charged biomolecule species at a specified location [3] [4] [5] . In this work, we showed that these devices can also efficiently separate biomolecules with different mobilities by focusing them at different locations. This phenomenon lends itself well to aptamer affinity probe CE, where aptamers undergo a significant mobility shift upon binding to larger target proteins. The important advantage of this scheme compared to conventional CE is that aptamer-protein dissociation and band broadening effects are counteracted by electrokinetic focusing. By simultaneously focusing and separating free aptamers from aptamer-protein complex in this device, we can obtain highly sensitive and quantitative measurement of target biomarkers using aptamers.
With this scheme, we showed enhanced detection sensitivity for IgE and HIV-1 RT in simple buffer solution. The limits of detection obtained (4.5 pM for IgE and 9 pM for HIV-1 RT) are among the lowest reported in the literature. The limit of detection for IgE in 10% serum was 10-fold higher due to nonspecific interactions between aptamers and serum proteins. Due to the simple readout for this assay, multiple samples can be assayed in parallel. As the assay is driven by gravitational flow, uses low voltages (30 V), and does not require multiple processing steps, it is well-suited towards low-cost point-of-care analysis.
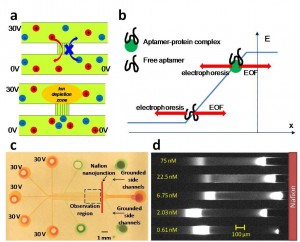
- Figure 1: a) Ion selective membrane creates a local ion-depletion zone with high electric field upon applying a voltage, b) Free aptamers and aptamer-protein complex concentrate at different locations on the electric field profile due to their different electrophoretic mobility, c) Optical image of multiplexd PDMS device with 200-µm-wide surface-patterned Nafion thin film on glass substrate. Sample channels and side channels are filled with red and green dyes, respectively. Experimental images are taken at the observation region, d) Multiplexed electrokinetic concentration enhanced aptamer mobility shift assay for detecting IgE in buffer solution.
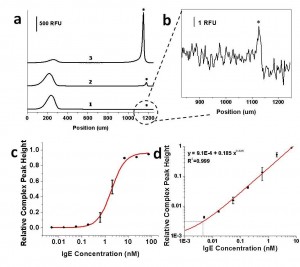
- Figure 2: a) Electropherogram for optimized electrokinetic concentration and separation of IgE aptamer (5nM) and different concentrations of IgE: (1) 4.92pM; (2) 0.6 nM, and (3) 6.75 nM IgE. The complex band is labeled with an asterisk; b) Inset demonstrates detection of 5 pM IgE in buffer; c) Dose-response curve of anti-IgE aptamer with IgE spiked in buffer, error bars represent standard error from duplicate experiments; d) Linear relationship in the log-log plot is obtained at low concentrations of IgE. The measured LOD is 4.4 pM IgE.
- I. German, D. D. Buchanan, and R. T. Kennedy, “Aptamers as ligands in affinity probe capillary electrophoresis,” Anal. Chem., vol. 70, pp. 4540-4545, 1998. [↩]
- H. Zhang, X. F. Li, and X. C. Le, “Tunable aptamer capillary electrophoresis and its application to protein analysis,” J. Am. Chem. Soc., vol. 130, pp. 34-35, 2008. [↩]
- Y. C. Wang, A. L. Stevens, and J. Han, “Million-fold preconcentration of proteins and peptides by nanofluidic filter,” Anal. Chem., vol. 77, pp. 4293-4299, 2005. [↩]
- Y. C. Wang and J. Han, “Pre-binding dynamic range and sensitivity enhancement for immuno-sensors using nanofluidic preconcentrator,” Lab on a Chip, vol. 8, pp. 392, 2008. [↩]
- L. F. Cheow, S. H. Ko, S. J. Kim, K. H. Kang, and J. Han, “Increasing the sensitivity of enzyme-linked immunosorbent assay using multiplexed electrokinetic concentrator,” Anal. Chem., vol. 82, pp. 3383-3388, 2010. [↩]