Continuous Microbioreactors
- Category: MEMS & BioMEMS
- Tags: Kevin Lee, Rajeev Ram
For systems biology, the models are more often limited by the absence of reliable experimental data than by available computational resources. Unfortunately, there is still great difficulty in making the leap from genetic and biochemical analysis to accurate verification with conventional culture growth experiments due to variations in culture conditions. Measurements of metabolic activity through substrate and product interactions or cellular activity through fluorescent interactions are highly dependent on environmental conditions and cellular metabolic state. For such experiments to be feasible, continuous cultures [1] [2] utilizing control strategies must be developed to measure chemical concentrations, introduce chemical inputs, and remove waste. An integrated microreactor system with built-in fluid metering will enable environmental control and programmable experiments capable of generating reproducible data.
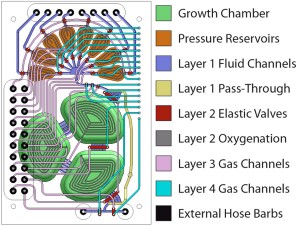
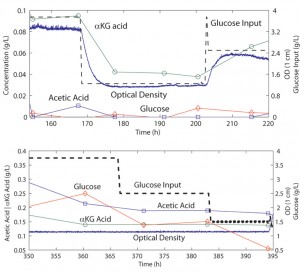
The chip shown in Figure 1 is fabricated out of a rigid plastic polycarbonate, utilizing PDMS membranes for actuation and pumping [3] . The fabrication process for bonding plastic-PDMS hybrid devices has been described previously [4] . Mixing and oxygen delivery are performed through membranes between the fluidic and actuation layers of the growth chamber sections. A growth volume of 1 mL ensures the ability to couple sampled volume to offline chemical analysis. Culture experiments are performed using E. coli strain FB21591 grown on defined media. The glucose input is separated to provide input control. As shown in Figure 2, various metabolic states are observable through continuous flow control. Cell density is directly dependent on glucose input, and acid production is proportional to cell density in chemostat mode. In turbidostat mode, cell density can be kept constant and glucose utilization can be observed, demonstrating the direct observation of overflow metabolism.
- Figure 1: Schematic of the micro-continuous culture device. Eight inputs lines provide control over the chemical composition of the medium within the growth reservoir. Fluids are introduced through a peristaltic pump, which injects liquid into the growth chamber.
- Figure 2: Plots of chemical concentrations in chemostat mode, demonstrating dependence of cell density on input glucose concentration. Plot of concentrations in turbidostat mode, demonstrating dependence of acetic acid on glucose concentration.
- J. Monod, “La technique de culture continue, theorie et applications,” Annales de I’Institut Pasteur, vol. 79, pp. 390-410, 1950. [↩]
- A. Novick and L. Szilard, “Description of the chemostat,” Science, vol. 112, pp. 715-716, 1950. [↩]
- V. Studer, G. Hang, A. Pandolfi, M. Ortiz, W. F. Anderson, and S. R. Quake, “Scaling properties of a low-actuation pressure microfluidic valve,” J. Applied Physics, vol. 95, pp. 393-398, 2004. [↩]
- K. S. Lee and R. J. Ram, “Plastic-PDMS bonding for high pressure hydrolytically stable active microfluidics,” Lab on a Chip, no. 9, pp. 1618-1624, 2009. [↩]