Cell-based Sensors for Measuring Impact of Microsystems on Cell Physiology
- Category: MEMS & BioMEMS
- Tags: Catherine Lo, Joel Voldman
The use of microsystems to manipulate and study cells in microenvironments is continually increasing. However, along with such increase in usage is also a growing concern regarding the impact of these microsystems on cell physiology. In this project, we are developing a set of cell-based fluorescent sensors to measure the impact of common stresses experienced in microsystems on cell physiology. We are including stress agents commonly found in microsystems (e.g., UV exposure, heat shock, fluid flow, etc.). Each sensor is designed to respond to one particular stress agent but can also be combined for multiplexed analysis of multiple stresses at once, as might be experienced in a typical microsystem. Designed to ease multiplexed analysis, each sensor will use different colors to both indicate the type of sensor and the strength of the signal.
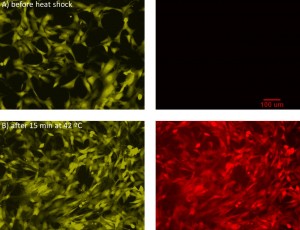
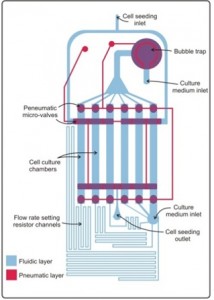
One sensor in the system will be a heat shock sensor that responds to activation of the heat shock pathway, which is a generalized stress pathway in cells. We are adapting a version of this sensor that we previously reported [1] [2] , which coupled fluorescent protein expression to activation of heat shock factor 1, from green fluorescent protein (EGFP) to a red fluorescent protein (RFP) and from red (DsRed) to yellow (YPet) for the constitutive color. Figure 1 shows the heat shock sensor response to 15 min heating at 42 ºC. Alongside this effort, we are using a multi-flow microfluidic device that can simultaneously apply different flows to cells across a 1000× range to understand the behavior of cells in flow [3] . Figure 2 is an image of the multi-flow device used to test NIH3T3 mouse fibroblast cells. Cells are seeded in 6 chambers concurrently and exposed to flow for 24 hrs, after which we can extract PCR from each chamber to study the cell response.
- Figure 1: Heat shock reporter sensor. Images of NIH3T3 cells (A) before and (B) after heat shock at 42 °C for 15 minutes, after recovering for 16 hrs. The left images are of the constitutive yellow reporter, while the right images are of the red fluorescent heat shock reporter.
- Figure 2: Multi-flow microfluidic device. Shown is a schematic of the multi-flowrate microfluidic device, showing the fluidic layer (blue) where the cells are grown and the pneumatic layer (red) containing microvalves.
- S. P. Desai and J. Voldman, “Cell-based sensors for quantifying the physiological impact of microsystems,” Integrative Biology, vol. 3, pp. 48-56, 2011. [↩]
- S. P. Desai and J. Voldman, “Measuring the impact of dielectrophoresis on cell physiology using a high-content screening platform,” Micro Total Analysis Systems ’08, San Diego, CA, USA, 2008, pp. 1308-10. [↩]
- Y.-C. Toh and J. Voldman, “Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction,” Faseb Journal, vol. 25, pp. 1208-17, 2011. [↩]