An Integrated Microfluidic Probe for Concentration-enhanced Selective Single Cell Kinase Activity Measurement
- Category: MEMS & BioMEMS
- Tags: Aniruddh Sarkar, Jongyoon Han
We present an integrated microfluidic probe that captures the contents of selected single adherent cells from standard tissue culture platforms and directly measures specific protein kinase activities in the captured lysate using either a fluorimetric assay in a small isolated chamber or a concentration-enhanced mobility-shift assay in an integrated nanofluidic concentrator. We demonstrate the use of the probe by measuring kinase activity in a single human hepatocellular carcinoma (HepG2) cell.
Traditional cellular assays measure average properties of 103-106 of cells, missing differences (e.g., drug responses) between individual cells in supposedly homogenous populations that have consequences for treatment of diseases [1] . Recent microfluidic or traditional tools [2] have studied genetic differences between single cells using nucleic acid amplification. These tools fail to capture important non-genetic sources of heterogeneity that create unique proteomes in different cells. Direct measurement of protein activities from single cells remains difficult due to limited assay sensitivity. In addition, difficulties in interfacing with adherent cells in a standard culture have led to the use of cell suspensions in microfluidic single cell assays [2] .
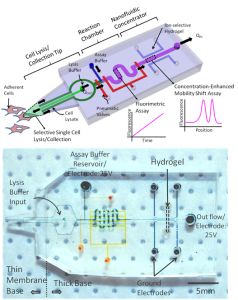
The integrated device (Figure 1) reported here interfaces with standard tissue culture plates using a microfluidic probe [3] that creates a limited, tunable lysis zone at its tip by simultaneously dispensing and collecting lysis agents and lyses and collects contents of selected single cells from adherent cell populations. The captured cytosol is mixed with assay reagents and flowed into a small reaction chamber, which is isolated for observation, using pneumatic micro-valves. The integrated ion-selective hydrogel-based nanofluidic concentrator [4] is then used to trap/concentrate the proteins/reaction products in the mixture to yield very high kinase assay sensitivity [5] , sufficient to probe proteins from single cells. This single cell detection platform is agnostic to specific sensing chemistry, so other biochemical assays can also be implemented with minimal modification.
- Figure 1: The integrated microfluidic probe is a 4-layer PDMS chip consisting of three modules: a cell lysis/collection tip to selectively lyse and collect single cells, a reaction chamber to mix and hold assay reagents, and a nanofluidic concentrator to concentrate product peptides and perform a mobility-shift assay for phosphorylated/unphosphorylated substrates.
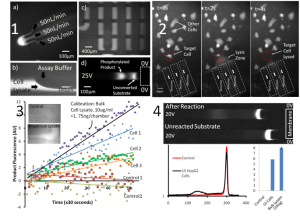
- Figure 2: i) Device operation showing lysis zone creation, mixing with assay reagents, capture of reaction mixture in a chamber with valves, and the concentration-enhanced mobility shift assay; ii) Single cell lysis while other cells are unaffected; iii) Measurement of protein kinase MK2 activity from single cells by a fluorimetric assay; iv) Measurement of protein kinase Akt activity in ~15 cells using a mobility shift assay.
- M. Niepel, S. L. Spencer, and P. K. Sorger, “Non-genetic cell-to-cell variability and the consequences for pharmacology,” Current Opinion in Chemical Biology, vol. 13, pp. 556-561, Dec. 2009. [↩]
- M. Leslie, “The Power of One,” Science, vol. 331, no. 6013, pp. 24-26, Jan. 2011. [↩] [↩]
- D. Juncker, H. Schmid, and E. Delamarche, “Multipurpose microfluidic probe,” Nature Materials, vol. 4, pp. 622-628, July 2005. [↩]
- Y.-C Wang, A. L. Stevens, and J. Han, “Million-fold preconcentration of proteins and peptides by nanofluidic filter,” Analytical Chemistry, vol. 77, no. 44, pp. 4293-4299, June 2005. [↩]
- J. H. Lee, B. D. Cosgrove, D. A. Lauffenburger, and J. Han, “Microfluidic concentration-cnhanced cellular kinase activity assay,” Journal of the American Chemical Society, vol. 131, no. 30, pp. 10340-10341, July 2009. [↩]