Paul Penfield,
Jr., Making Complexity Simple,
Complexity in Engineering Conference, Cambridge, MA; November 20, 1999.
Making Complexity
Simple
Making Complexity Simple
- Outline
- Arcane
- Pity
- Perspective
- Information
- Course
- Status
The Second Law of Thermodynamics
Surely one of science's most glorious accomplishments
 Also one of the most profound and mysterious
Also one of the most profound and mysterious
 What is this thing called "entropy?"
What is this thing called "entropy?"
 Does the Second Law really tell us which way clocks run?
Does the Second Law really tell us which way clocks run?
 Deep related concepts
Deep related concepts

 Complexity
Complexity

 Randomness
Randomness

 Fluctuations
Fluctuations

 Dissipation
Dissipation

 Order
Order
 The public's imagination is captured
The public's imagination is captured
Nobody really understands it
 At least that is what we believed as graduate students
At least that is what we believed as graduate students
 It is too complex to be taught
It is too complex to be taught
Why is entropy considered arcane?
Because people think it is part of thermodynamics
 Thermodynamics really is hard to understand.
Thermodynamics really is hard to understand.

 Many concepts -- heat, work, temperature, ...
Many concepts -- heat, work, temperature, ...

 You have to care about things like ideal gases.
You have to care about things like ideal gases.
 Most engineers don't need to know thermodynamics.
Most engineers don't need to know thermodynamics.
 Most don't care.
Most don't care.
This is a shame
Entropy and the Second Law are taught as part of
thermodynamics, so most people miss them.
... and thereby miss something pretty important
 All scientists, all engineers, indeed all educated people need
All scientists, all engineers, indeed all educated people need
 to understand that some operations are reversible and
to understand that some operations are reversible and
 some are not.
some are not.
 They need to be able to tell them apart.
They need to be able to tell them apart.
 They need to know there is a way to quantify reversibility.
They need to know there is a way to quantify reversibility.
 They need a "monotonic model"
They need a "monotonic model"

 To complement models of conserved quantities
To complement models of conserved quantities

 Both are helpful for understanding the world
Both are helpful for understanding the world

 Both are prototypes for other quantities
Both are prototypes for other quantities
Can we satisfy these very real needs somehow?
They have nothing to do with thermodynamics.
 It's just that thermodynamics is where they have traditionally
It's just that thermodynamics is where they have traditionally
 been taught.
been taught.
Entropy is useful outside of thermodynamics
Thermodynamics always involves energy
 Entropy need not
Entropy need not
Outside of thermodynamics, without links to energy
 Entropy is less complex
Entropy is less complex
 There are plenty of reversible and irreversible operations
There are plenty of reversible and irreversible operations
 The Second Law, or something much like it, exists
The Second Law, or something much like it, exists
 Monotonic models can be taught more easily
Monotonic models can be taught more easily
The more general the context, the simpler the concept
 This is the secret of making complexity simple
This is the secret of making complexity simple
War is too important to be left to the generals*
And entropy is too important to be left to the physicists
 * George Clémenceau (1841 - 1929)
* George Clémenceau (1841 - 1929)

 French Premier, 1906 - 1909, 1917 - 1920
French Premier, 1906 - 1909, 1917 - 1920
Information is simpler and more general
Start with information
 Entropy is one kind of information.
Entropy is one kind of information.
 Entropy is information we do not have
Entropy is information we do not have
See reversible and irreversible data transformations
 In computation and communications
In computation and communications
Note that irreversible operations destroy information
 This is the Second Law in this context
This is the Second Law in this context
Apply to a physical system with energy
Use maximum-entropy principle
 Voila, thermodynamics!
Voila, thermodynamics!
 Temperature is energy per bit of entropy (sort of)
Temperature is energy per bit of entropy (sort of)
 Intensive vs. extensive variables
Intensive vs. extensive variables
 Second Law in traditional setting
Second Law in traditional setting
 Carnot efficiency
Carnot efficiency
The basic idea of reversibility is not difficult to understand
We want to teach this stuff to freshmen
Why is this possible?
 Today's students are different
Today's students are different
 Best to start from the known
Best to start from the known

 Data, disks, Internet, packets, bits, ...
Data, disks, Internet, packets, bits, ...

 Consistent with the coming information age
Consistent with the coming information age
 Go toward the unknown
Go toward the unknown

 Thermodynamics, equilibrium, heat engines, refrigerators
Thermodynamics, equilibrium, heat engines, refrigerators

 Relevant to the current industrial age
Relevant to the current industrial age
 Physical view of information . . . like energy, information
Physical view of information . . . like energy, information

 can be of many types
can be of many types

 can be converted from one form to another
can be converted from one form to another

 can exist in one place or another
can exist in one place or another

 can be sent from here to there
can be sent from here to there

 can be stored for later use
can be stored for later use
 There are interesting applications
There are interesting applications

 Biology (genetic code)
Biology (genetic code)

 Communications
Communications

 Quantum computing
Quantum computing
Information and Entropy
A Freshman Course
 12 weeks:
12 weeks:

 1. Bits
1. Bits

 2. Codes
2. Codes

 3. Compression
3. Compression

 4. Errors
4. Errors

 5. Probability
5. Probability

 6. Communications
6. Communications

 7. Processes
7. Processes

 8. Inference
8. Inference

 9. Entropy
9. Entropy

 10. Physical systems
10. Physical systems

 11. Temperature
11. Temperature

 12. Myths
12. Myths
This course is NOT
 Introduction to Computing
Introduction to Computing
 Introductory Communications
Introductory Communications
 Thermodynamics 101
Thermodynamics 101
Course material
1. Bits
 Restoring logic
Restoring logic
 Digital abstraction
Digital abstraction
 Signals and streams
Signals and streams
 Boolean algebra
Boolean algebra
2. Codes
 Bytes
Bytes
 Fixed-length codes
Fixed-length codes

 ASCII
ASCII

 Genetic code
Genetic code

 Binary code, gray code
Binary code, gray code
 Variable-length codes
Variable-length codes

 Morse code
Morse code

 Telegraph codebooks
Telegraph codebooks
Course material (cont)
3. Compression
 Helps with low channel capacity
Helps with low channel capacity
 Codebooks
Codebooks
 Irreversible -- fidelity requirement
Irreversible -- fidelity requirement

 JPEG
JPEG

 MP3
MP3
 Reversible
Reversible

 Run length encoding
Run length encoding

 LZW
LZW

 The LZW patent issue
The LZW patent issue
4. Errors
 Physical sources of noise
Physical sources of noise
 Detection -- parity
Detection -- parity
 Correction
Correction

 Triple redundancy
Triple redundancy

 Hamming code
Hamming code
Course material (cont)
5. Probability
 Racing odds
Racing odds
 Random sources
Random sources

 coins, dice, cards
coins, dice, cards
 Probabilities are subjective
Probabilities are subjective
 Information can be quantified
Information can be quantified
6. Communications
 Model with source, coder, channel, decoder, receiver
Model with source, coder, channel, decoder, receiver
 Huffman codes
Huffman codes
 Symmetric binary channel
Symmetric binary channel

 Lossless
Lossless

 Noisy
Noisy
 Client-server model
Client-server model

 TCP and IP
TCP and IP

 Strategies for recovery from lost packets
Strategies for recovery from lost packets
Course material (cont)
7. Processes
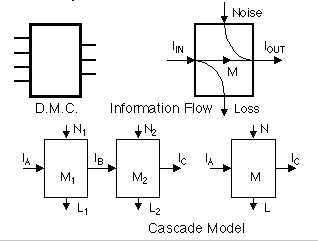
 Discrete memoryless channel
Discrete memoryless channel
 Noise, loss
Noise, loss
 M = IIN - L
M = IIN - L

 = IOUT - N
= IOUT - N
 Cascade inequalities in L, N, and M
Cascade inequalities in L, N, and M



 L1 <= L
L1 <= L

 L1 + L2 - N1 <= L
<= L1 + L2
L1 + L2 - N1 <= L
<= L1 + L2
8. Inference
 Given received signal, what is input
Given received signal, what is input
 How much information have we learned?
How much information have we learned?
Course material (cont)
Discrete Memoryless Channel
Course material (cont)
9. Entropy
 Entropy is information we do not have
Entropy is information we do not have
 Input probabilities consistent with constraints
Input probabilities consistent with constraints
 Minimum assumptions, maximum entropy
Minimum assumptions, maximum entropy
 Lagrange multipliers
Lagrange multipliers

 Deer hunters
Deer hunters

 Fishermen
Fishermen
10. Physical systems
 Energy per state
Energy per state
 Expected value of energy
Expected value of energy
 Boltzmann distribution
Boltzmann distribution
 Lagrange multipliers are intensive variables
Lagrange multipliers are intensive variables
 Equilibrium
Equilibrium
Course material (cont)
11. Temperature
 One of the Lagrange multipliers is temperature
One of the Lagrange multipliers is temperature
 Heat, work
Heat, work
 Carnot efficiency
Carnot efficiency
12. Myths
 Order out of chaos
Order out of chaos
 Miracle needed
Miracle needed
 Heat death
Heat death
 Evaporation of black holes
Evaporation of black holes
 Difficulty of extensions to social science
Difficulty of extensions to social science
Can freshmen actually learn this stuff?
We are finding out
 Fall 1999, course development
Fall 1999, course development

 Faculty: Paul Penfield, Seth Lloyd, Sherra Kerns
Faculty: Paul Penfield, Seth Lloyd, Sherra Kerns

 Students: small set of freshmen serving as guinea pigs
Students: small set of freshmen serving as guinea pigs
 Spring 2000, pilot offering
Spring 2000, pilot offering

 Limited to 50 freshmen
Limited to 50 freshmen

 Of course we have a Web site -- everybody does
Of course we have a Web site -- everybody does

 https://mtlsites.mit.edu/users/penfield/6.095-s00/
https://mtlsites.mit.edu/users/penfield/6.095-s00/
 Fall 2000, revisions, note writing
Fall 2000, revisions, note writing
 Spring 2001, first full offering
Spring 2001, first full offering
The devil is in the details
 E.g., which is the best statistical mechanics model to use?
E.g., which is the best statistical mechanics model to use?
Some of the subtle points (risks)
Entropy is the information we don't have
 Therefore entropy is subjective (some people don't like that)
Therefore entropy is subjective (some people don't like that)
Math generally simple -- discrete, not continuous processes
 But Lagrange multipliers are not easy
But Lagrange multipliers are not easy
Skill in modeling is still important
 No magic here
No magic here
At the freshman level you cannot go very deeply
 All we can do is provide simple ideas to be built on later
All we can do is provide simple ideas to be built on later

 We want to be consistent with later learning in specific areas
We want to be consistent with later learning in specific areas
Stay in touch -- we will let you know how it turns out
If we are successful
 The course will be permanent
The course will be permanent
 We will help other universities start similar courses
We will help other universities start similar courses
 We will advocate it as a science exposure in the liberal arts
We will advocate it as a science exposure in the liberal arts
URL of this page:
https://mtlsites.mit.edu/users/penfield/pubs/complex-99.html
Created: Nov 19, 1999 |
Modified: Dec 29, 1999
Related page: Penfield publication list
Site map |
To Paul Penfield's home page |
Your comments are welcome.
Click here for information on MIT Accessibility